Novel Product Candidates for the Treatment of Neurological Diseases
With a deep commitment to scientific discovery and translation, we pursue any path necessary to target key biochemical checkpoints to disrupt the disease process. Our current focus is the analysis and disruption of neurodegenerative pathways in the brain and gastrointestinal tract that lead to Parkinson’s and related diseases.
Key technologies include:
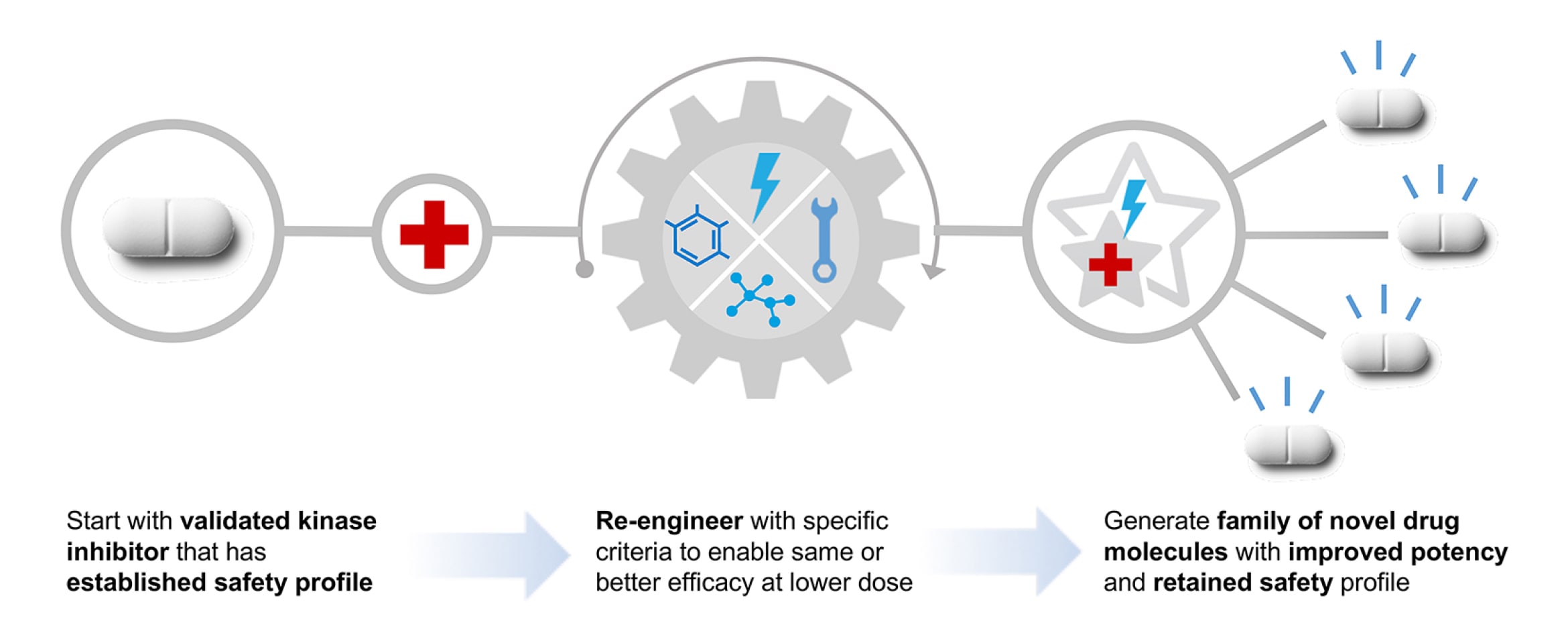
- RAMP™: Re-engineering Approach with Metabolism Preserved, a method of designing novel medications by learning from clinically evaluated and/or marketed drug products to build in desirable, clinically validated safety or pharmacology characteristics from the template into new drug substances.
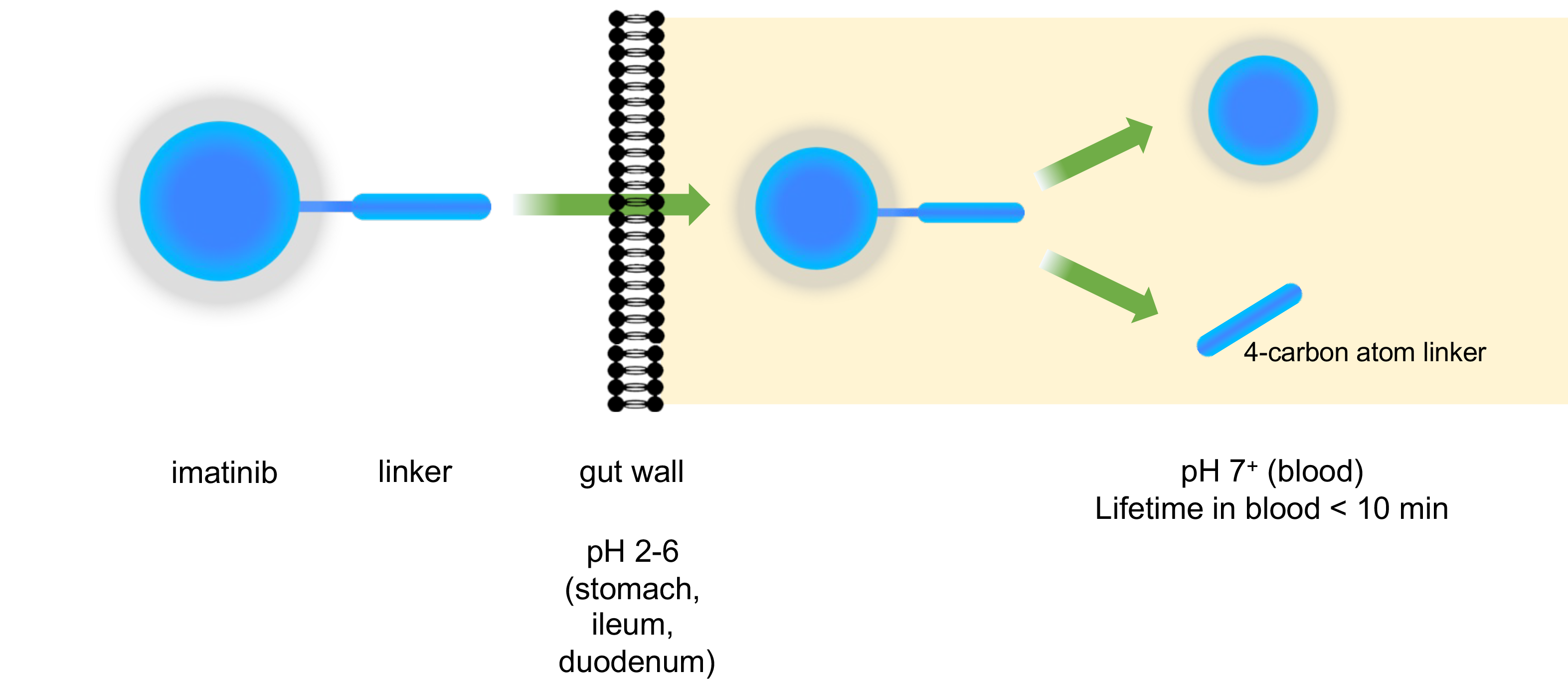
- Prodrug delivery methods: Novel prodrug linkers that enhance drug absorption, delivery and/or suppress undesired on-dosing side effects.
RAMP™ Enables the Discovery of Novel Candidates
Treating Parkinson’s and Related Diseases with Small-Molecule Kinase Inhibitors
We are utilizing our RAMP™ discovery engine to develop a new class of protein kinase inhibitors for the potential treatment of kinase-sensitive CNS diseases. These candidates could be more potent and safer than commercially marketed kinase inhibitors and be administered chronically and systemically.
Additional Compounds
We are actively developing novel kinase inhibitor treatments for additional Parkinson’s-related disorders, including multiple system atrophy (MSA), a neurodegenerative disorder that shares the hallmarks of c-Abl activation and alpha-synuclein pathology with Parkinson’s disease. Future programs will include Dementia with Lewy Body (DLB), a Parkinson’s-like disease characterized by alpha-synuclein aggregates that primarily lead to cognitive decline without a significant movement disorder component.

Intellectual Property
Our intellectual property includes patents issued or pending, as well as know-how related to the composition of matter and broad use of our technologies across neurodegenerative diseases inside and outside of the brain. We have carved out a broad and deep intellectual property position that enables the development of the Company’s internal product pipeline and supports value-creating partnerships with biotechnology and pharmaceutical companies.