

Exhibit 99.1 Corporate Overview November 2025

Disclaimer This presentation contains information that may constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Inhibikase Therapeutics, Inc. (the “Company” or “we”) intends for the forward- looking statements to be covered by the safe harbor provisions for forward-looking statements in those sections. Generally, we have identified such forward-looking statements by using the words “believe,” “expect,” “intend,” “estimate,” “anticipate,” “project,” “target,” “forecast,” “aim,” should, “will,” may”, “continue”, “assume”, “contemplate”, “could”, “design”, “due”, “goal”, “hope”, “might”, “plan”, “opportunity”, “predict”, “possible”, “potential”, “seek”, “strategy”, “would” and similar expressions that are predictions or indicate future events and future trends, or the negative of these terms or other comparable terminology. Such statements are subject to a number of assumptions, risks and uncertainties which may cause actual results, performance or achievements to be materially different from those anticipated in these forward-looking statements. You should read statements that contain these words carefully because they discuss future expectations and plans which contain projections of future clinical studies or trials, regulatory approvals, product candidate development, results of operations or financial condition or market opportunity or state other forward-looking information. However, the absence of these words or similar expressions does not mean that a statement is not forward-looking. Forward-looking statements are not historical facts, but instead represent only the Company’s beliefs regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company’s control. It is possible that the Company’s actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Management believes that these forward-looking statements are reasonable as of the time made. However, caution should be taken not to place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from the Company's historical experience and our present expectations or projections. This presentation should be read in conjunction with the Company’s filings with the Securities and Exchange Commission, including its annual report on Form 10-K, its quarterly Form 10-Q and any subsequent filings with the SEC (collectively, the “SEC Filings”). Important factors that could cause actual results to differ materially from those in the forward- looking statements include, but are not limited to, our ability to execute clinical trials, including to evaluate IKT-001 as a treatment for pulmonary arterial hypertension, as well as such other factors that are set forth in the Company’s SEC filings, including under the caption Risk Factors”. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified , and make no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. In addition, no independent sources has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation related to or based on such internal estimates and research. We do not intend our use or display of other entities’ names, trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity. This presentation shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. 2

Experienced Leadership with Deep Expertise in PAH MARK IWICKI CHRIS CABELL, MD MHS FACC DAVID McINTYRE BEC CPA LLB MBA JEFF KAGY Chief Executive Officer Head of R&D, Chief Medical Officer Chief Financial Officer Chief Human Resource Officer JOHN ADAMS, PHD TIM PIGOT CHAD OREVILLO, MPH ALLISON WIDLITZ, MS, PA Chief Scientific Officer Chief Commercial Officer EVP, Development Operations VP, Clinical Development 3
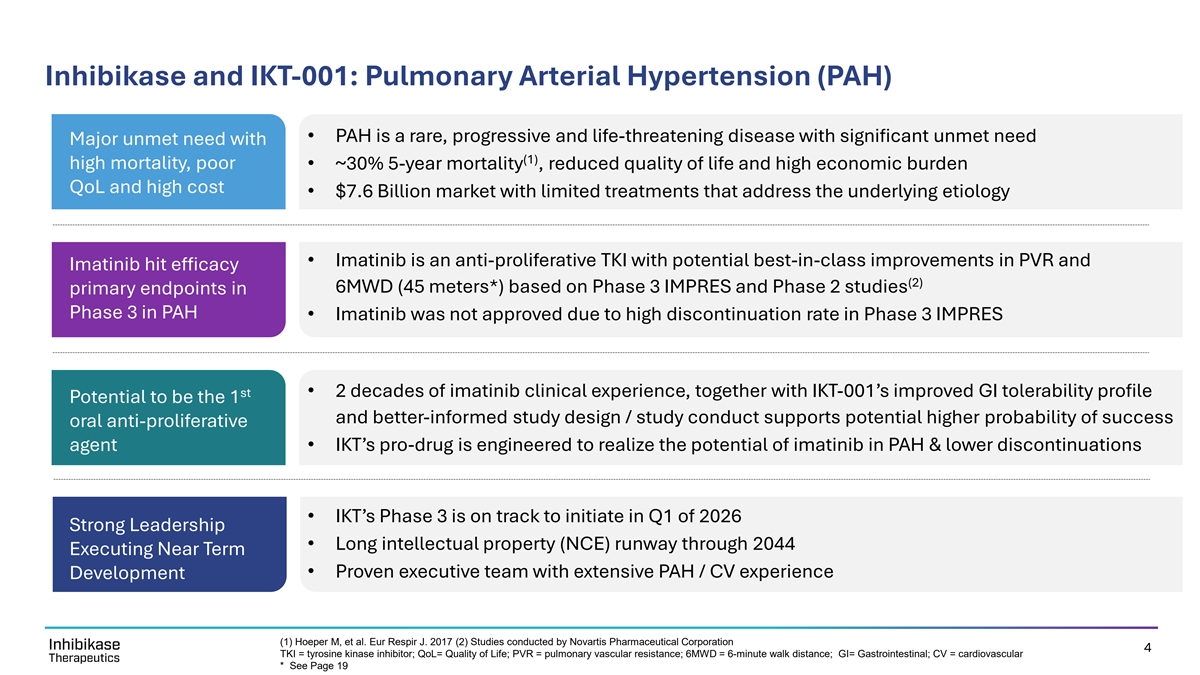
Inhibikase and IKT-001: Pulmonary Arterial Hypertension (PAH) • PAH is a rare, progressive and life-threatening disease with significant unmet need Major unmet need with (1) high mortality, poor • ~30% 5-year mortality , reduced quality of life and high economic burden QoL and high cost • $7.6 Billion market with limited treatments that address the underlying etiology • Imatinib is an anti-proliferative TKI with potential best-in-class improvements in PVR and Imatinib hit efficacy (2) 6MWD (45 meters*) based on Phase 3 IMPRES and Phase 2 studies primary endpoints in Phase 3 in PAH • Imatinib was not approved due to high discontinuation rate in Phase 3 IMPRES st • 2 decades of imatinib clinical experience, together with IKT-001’s improved GI tolerability profile Potential to be the 1 and better-informed study design / study conduct supports potential higher probability of success oral anti-proliferative agent • IKT’s pro-drug is engineered to realize the potential of imatinib in PAH & lower discontinuations • IKT’s Phase 3 is on track to initiate in Q1 of 2026 Strong Leadership • Long intellectual property (NCE) runway through 2044 Executing Near Term • Proven executive team with extensive PAH / CV experience Development (1) Hoeper M, et al. Eur Respir J. 2017 (2) Studies conducted by Novartis Pharmaceutical Corporation 4 TKI = tyrosine kinase inhibitor; QoL= Quality of Life; PVR = pulmonary vascular resistance; 6MWD = 6-minute walk distance; GI= Gastrointestinal; CV = cardiovascular * See Page 19

PAH is a Progressive Disease Driven by Uncontrolled Cell Proliferation Proliferation of vascular cells drive vascular remodeling, raising pulmonary artery pressure and leading to progressive right ventricular heart failure and ultimately death 5 PASMCs = Pulmonary artery smooth muscle cells; Vascular smooth muscle cells 5
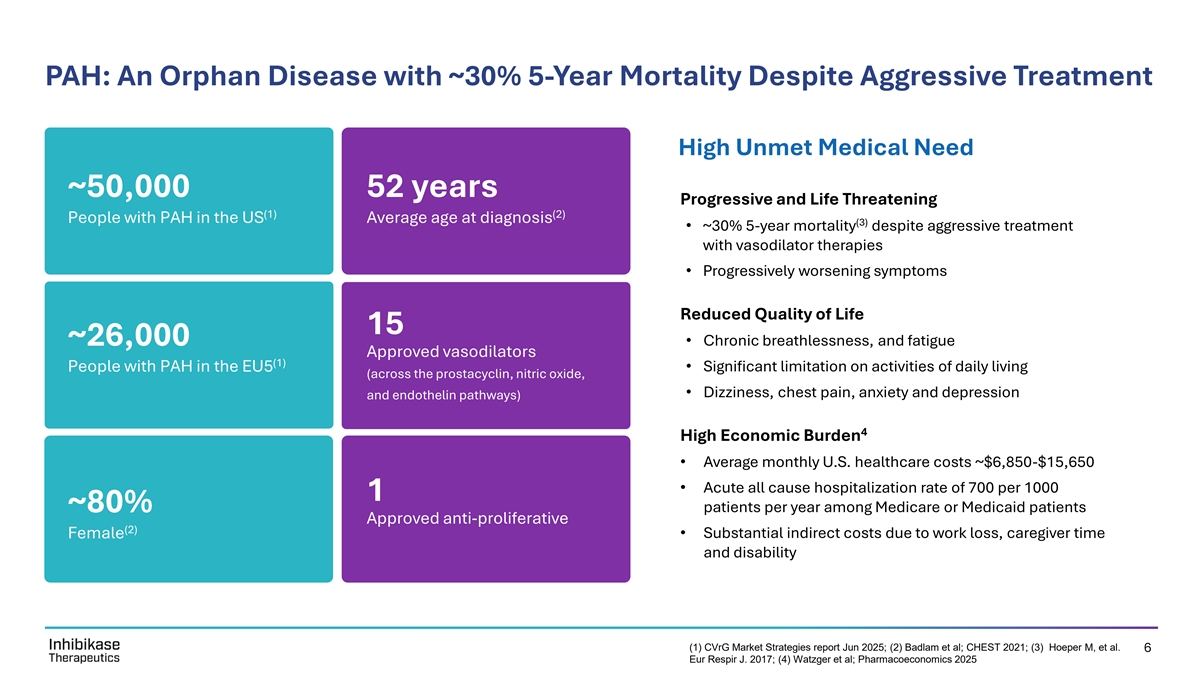
PAH: An Orphan Disease with ~30% 5-Year Mortality Despite Aggressive Treatment High Unmet Medical Need ~50,000 52 years Progressive and Life Threatening (1) (2) People with PAH in the US Average age at diagnosis (3) • ~30% 5-year mortality despite aggressive treatment with vasodilator therapies • Progressively worsening symptoms Reduced Quality of Life 15 ~26,000• Chronic breathlessness, and fatigue Approved vasodilators (1) People with PAH in the EU5• Significant limitation on activities of daily living (across the prostacyclin, nitric oxide, • Dizziness, chest pain, anxiety and depression and endothelin pathways) 4 High Economic Burden • Average monthly U.S. healthcare costs ~$6,850-$15,650 • Acute all cause hospitalization rate of 700 per 1000 1 ~80% patients per year among Medicare or Medicaid patients Approved anti-proliferative (2) Female• Substantial indirect costs due to work loss, caregiver time and disability (1) CVrG Market Strategies report Jun 2025; (2) Badlam et al; CHEST 2021; (3) Hoeper M, et al. 6 Eur Respir J. 2017; (4) Watzger et al; Pharmacoeconomics 2025
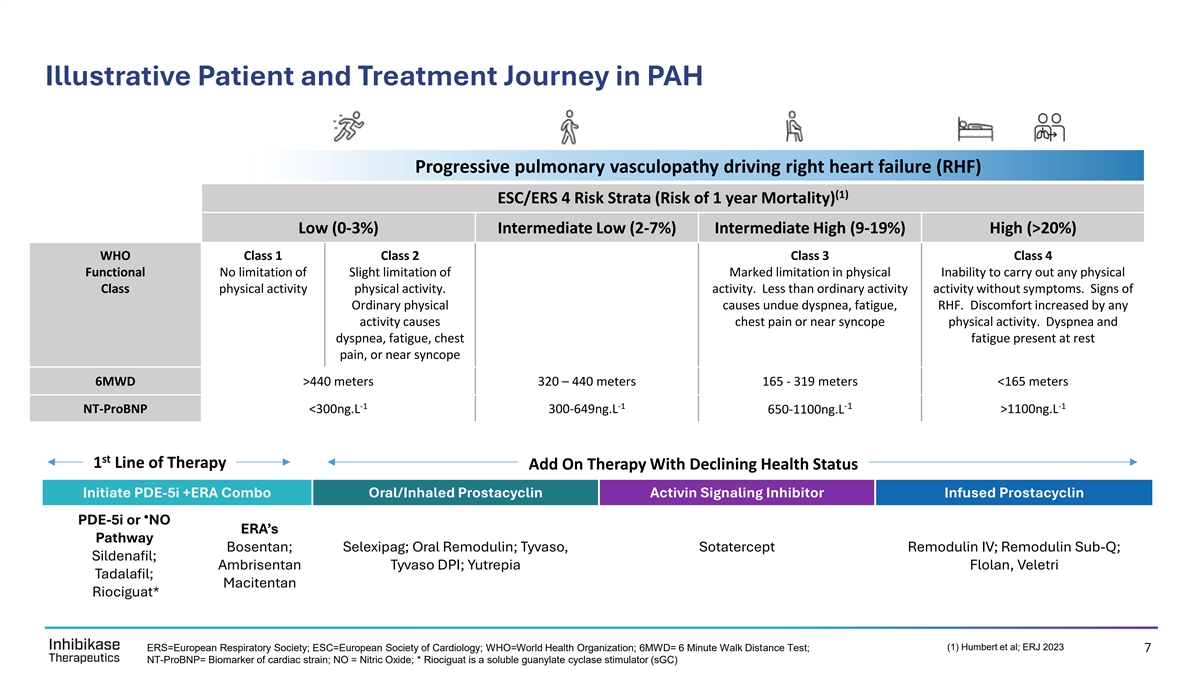
Illustrative Patient and Treatment Journey in PAH Progressive pulmonary vasculopathy driving right heart failure (RHF) (1) ESC/ERS 4 Risk Strata (Risk of 1 year Mortality) Low (0-3%) Intermediate Low (2-7%) Intermediate High (9-19%) High (>20%) WHO Class 1 Class 2 Class 3 Class 4 Functional No limitation of Slight limitation of Marked limitation in physical Inability to carry out any physical Class physical activity physical activity. activity. Less than ordinary activity activity without symptoms. Signs of Ordinary physical causes undue dyspnea, fatigue, RHF. Discomfort increased by any activity causes chest pain or near syncope physical activity. Dyspnea and dyspnea, fatigue, chest fatigue present at rest pain, or near syncope 6MWD >440 meters 320 – 440 meters 165 - 319 meters <165 meters -1 -1 -1 -1 NT-ProBNP <300ng.L 300-649ng.L 650-1100ng.L >1100ng.L st 1 Line of Therapy Add On Therapy With Declining Health Status Initiate PDE-5i +ERA Combo Oral/Inhaled Prostacyclin Activin Signaling Inhibitor Infused Prostacyclin • PDE-5i or NO ERA’s Pathway Bosentan; Selexipag; Oral Remodulin; Tyvaso, Sotatercept Remodulin IV; Remodulin Sub-Q; Sildenafil; Ambrisentan Tyvaso DPI; Yutrepia Flolan, Veletri Tadalafil; Macitentan Riociguat* CONFIDENTIAL (1) Humbert et al; ERJ 2023 ERS=European Respiratory Society; ESC=European Society of Cardiology; WHO=World Health Organization; 6MWD= 6 Minute Walk Distance Test; 7 NT-ProBNP= Biomarker of cardiac strain; NO = Nitric Oxide; * Riociguat is a soluble guanylate cyclase stimulator (sGC)
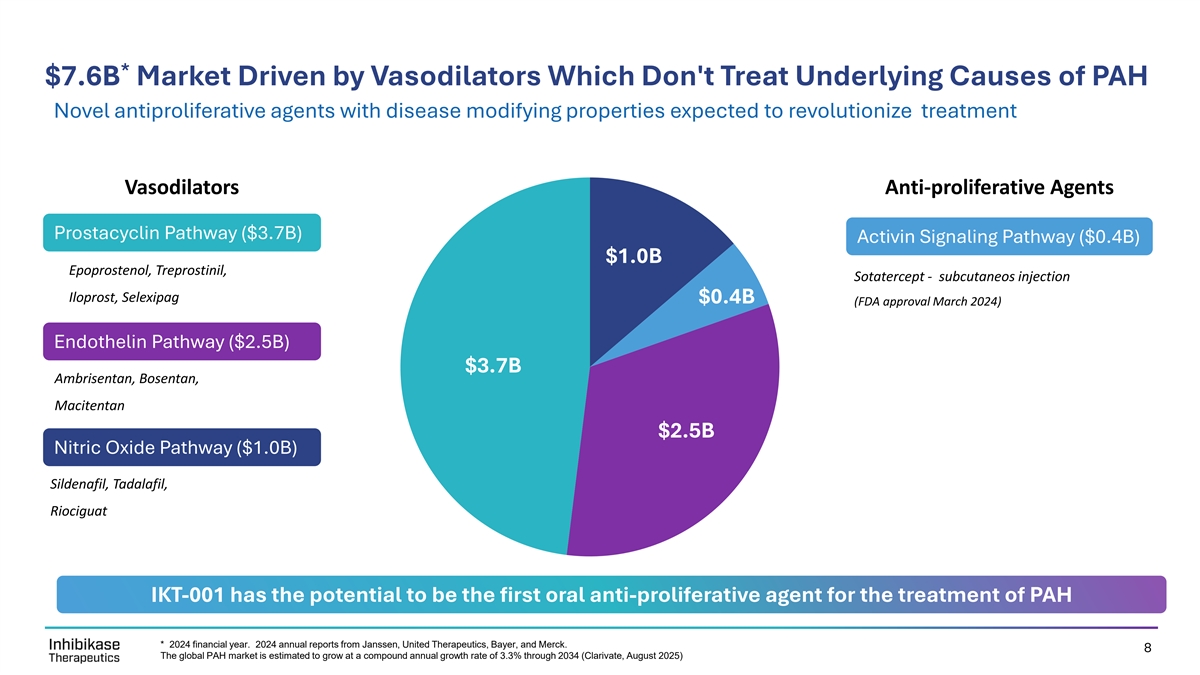
* $7.6B Market Driven by Vasodilators Which Don't Treat Underlying Causes of PAH Novel antiproliferative agents with disease modifying properties expected to revolutionize treatment Vasodilators Anti-proliferative Agents Prostacyclin Pathway ($3.7B) Activin Signaling Pathway ($0.4B) $1.0B $1.0B Epoprostenol, Treprostinil, Sotatercept - subcutaneos injection $0.4B Iloprost, Selexipag $0.4B (FDA approval March 2024) Endothelin Pathway ($2.5B) $3.7B $3.7B Ambrisentan, Bosentan, Macitentan $2.5B $2.5B Nitric Oxide Pathway ($1.0B) Sildenafil, Tadalafil, Riociguat IKT-001 has the potential to be the first oral anti-proliferative agent for the treatment of PAH * 2024 financial year. 2024 annual reports from Janssen, United Therapeutics, Bayer, and Merck. 8 The global PAH market is estimated to grow at a compound annual growth rate of 3.3% through 2034 (Clarivate, August 2025)

Our Solution: An Oral Pro-Drug of Imatinib Optimized for PAH Engineered to realize imatinib’s best-in-class efficacy potential in PAH Gleevec IKT-001 (Imatinib) (Imatinib Pro-Drug) History IKT-001 is an investigational novel pro-drug of imatinib First Approved in 2001; 25 years of real-world experience designed for better GI tolerability to support optimal Indicated for: Leukemia, Soft Tissue Sarcoma, Myelodysplastic Syndromes, Mastocytosis and GIST efficacy PAH Data Best-in-class improvements in PVR and 6MWD in Phase 2 & 3 Potential to be the first and only once-daily oral anti-proliferative but not approved due to high discontinuations. Contemporary tyrosine kinase inhibitor (TKI) for PAH imatinib study* reinforces imatinib efficacy potential and supports improved discontinuation profile * Rothman et al., AJRCCM 2025. PVR = Pulmonary Vascular Resistance; 6MWD= 6 Minute Walk Distance test; 9 GI = Gastrointestinal; GIST = Gastrointestinal Stromal Tumor

IKT-001
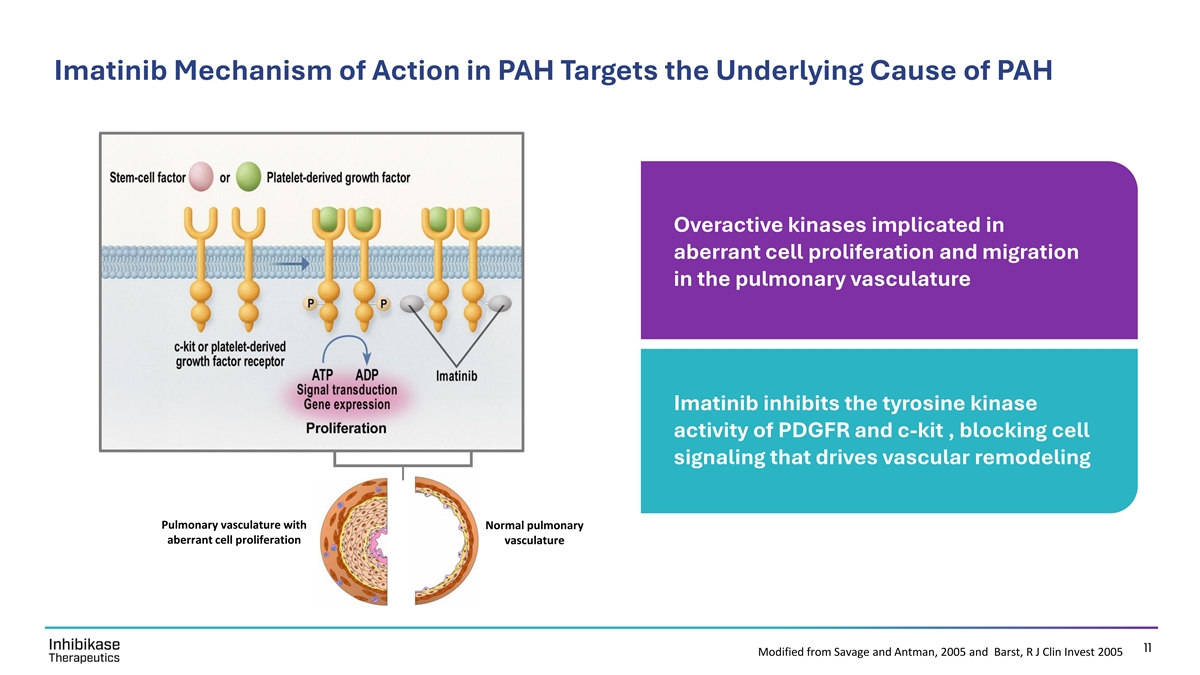
Imatinib Mechanism of Action in PAH Targets the Underlying Cause of PAH Overactive kinases implicated in aberrant cell proliferation and migration in the pulmonary vasculature Imatinib inhibits the tyrosine kinase activity of PDGFR and c-kit , blocking cell signaling that drives vascular remodeling Pulmonary vasculature with Normal pulmonary aberrant cell proliferation vasculature 11 Modified from Savage and Antman, 2005 and Barst, R J Clin Invest 2005

Imatinib Demonstrated Reversal of PAH in Standard Animal Model* Improved Reversal of Reversal of increased right vascular remodeling ventricular pressure survival Day 0 MCT d42 MCT d42/+imatinib Imatinib reverses pulmonary vascular remodeling, right ventricular pressure / remodeling and improves survival STI571 = imatinib; MCT = monocrotaline †‡ *P < 0.05 versus control; P < 0.05 versus MCT at day 28 or hypoxia at day 21; P < 0.05 versus MCT at day 42 or hypoxia at day 35. * Schermuly et al., JCI 2005 12

IKT-001 Minimizes* GI Imatinib Exposure to Drive Increased Tolerability 28 Day non-human primate study documents improved GI tolerability blood imatinib IKT-001 >2.5x Improvement in GI Tolerability enzymatic IKT-001 cleavage Dose Associated with GI Toxicity Imatinib: 75 mg per kg per day IKT-001: 200 mg per kg per day Pro-drug linker gut gut wall GI = gastrointestinal 13 * In preclinical studies
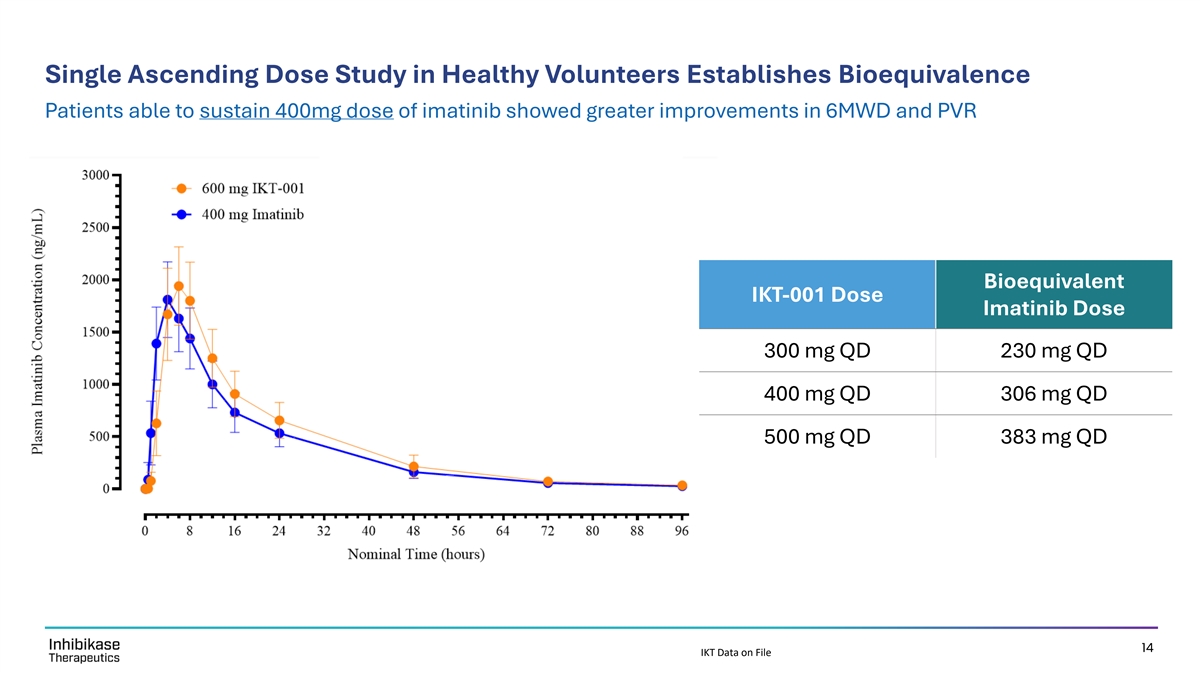
Single Ascending Dose Study in Healthy Volunteers Establishes Bioequivalence Patients able to sustain 400mg dose of imatinib showed greater improvements in 6MWD and PVR Bioequivalent IKT-001 Dose Imatinib Dose 300 mg QD 230 mg QD 400 mg QD 306 mg QD 500 mg QD 383 mg QD 14 IKT Data on File

Clinical Rationale
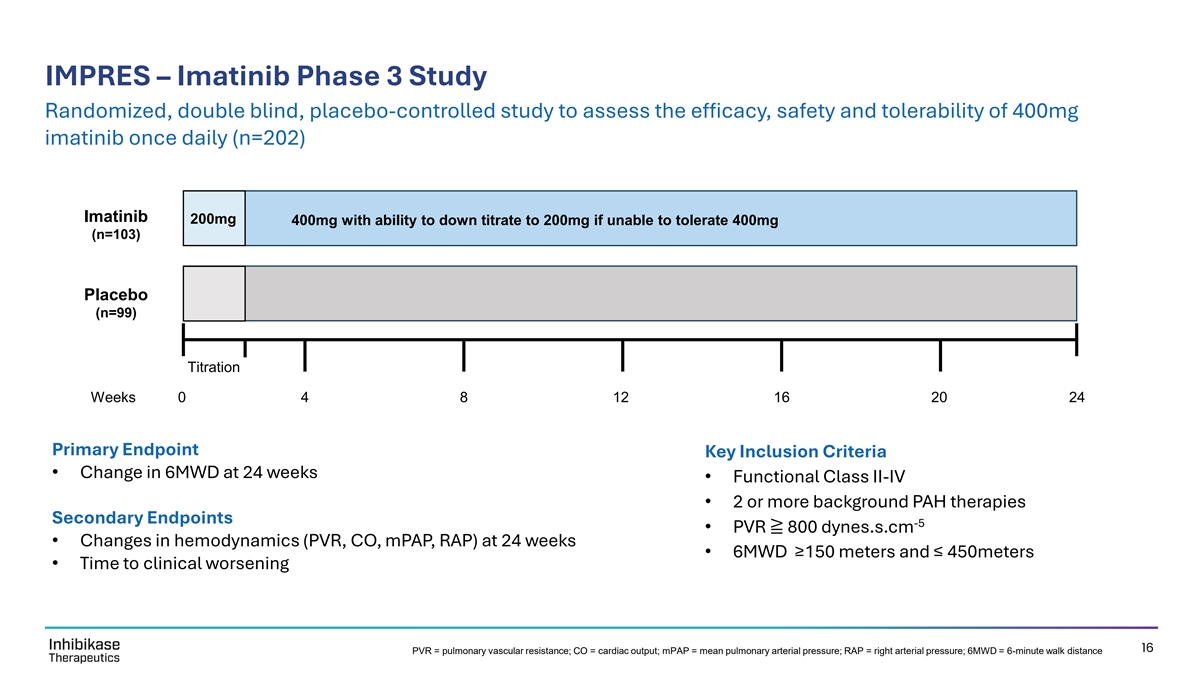
IMPRES – Imatinib Phase 3 Study Randomized, double blind, placebo-controlled study to assess the efficacy, safety and tolerability of 400mg imatinib once daily (n=202) Imatinib 200mg 400mg with ability to down titrate to 200mg if unable to tolerate 400mg (n=103) Placebo (n=99) Titration Weeks 0 4 8 12 16 20 24 Primary Endpoint Key Inclusion Criteria • Change in 6MWD at 24 weeks • Functional Class II-IV • 2 or more background PAH therapies Secondary Endpoints -5 • PVR ≧ 800 dynes.s.cm • Changes in hemodynamics (PVR, CO, mPAP, RAP) at 24 weeks • 6MWD ≥150 meters and ≤ 450meters • Time to clinical worsening 16 PVR = pulmonary vascular resistance; CO = cardiac output; mPAP = mean pulmonary arterial pressure; RAP = right arterial pressure; 6MWD = 6-minute walk distance
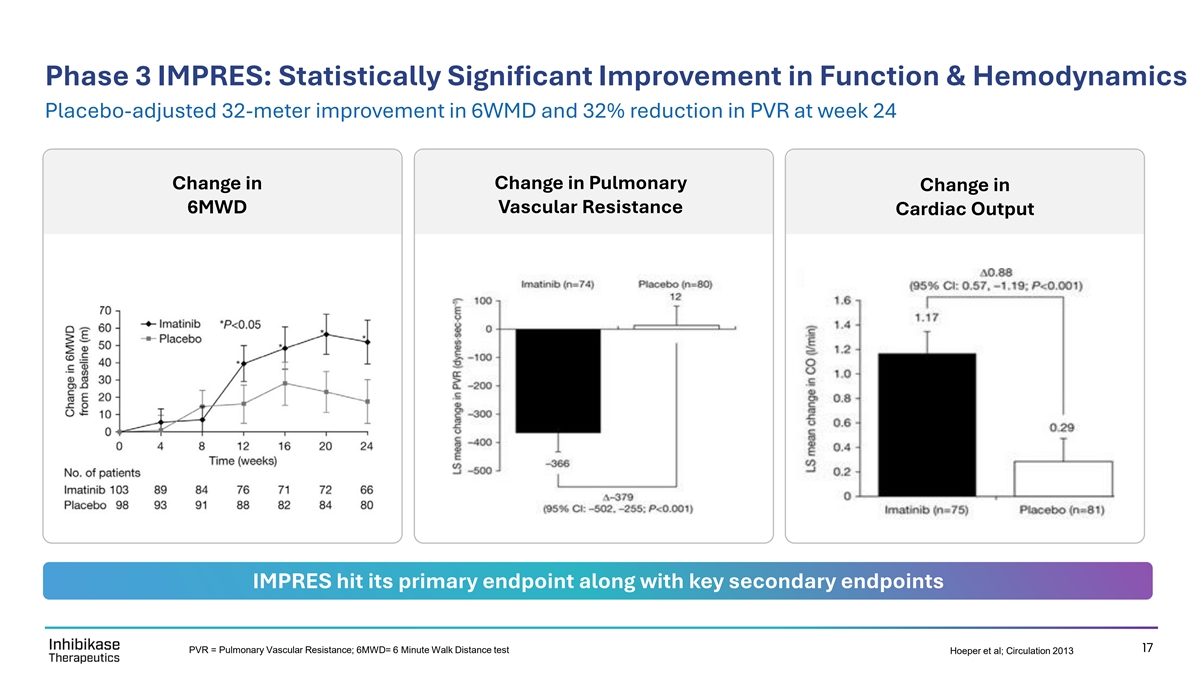
Phase 3 IMPRES: Statistically Significant Improvement in Function & Hemodynamics Placebo-adjusted 32-meter improvement in 6WMD and 32% reduction in PVR at week 24 Change in Pulmonary Change in Change in 6MWD Vascular Resistance Cardiac Output IMPRES hit its primary endpoint along with key secondary endpoints 17 PVR = Pulmonary Vascular Resistance; 6MWD= 6 Minute Walk Distance test Hoeper et al; Circulation 2013
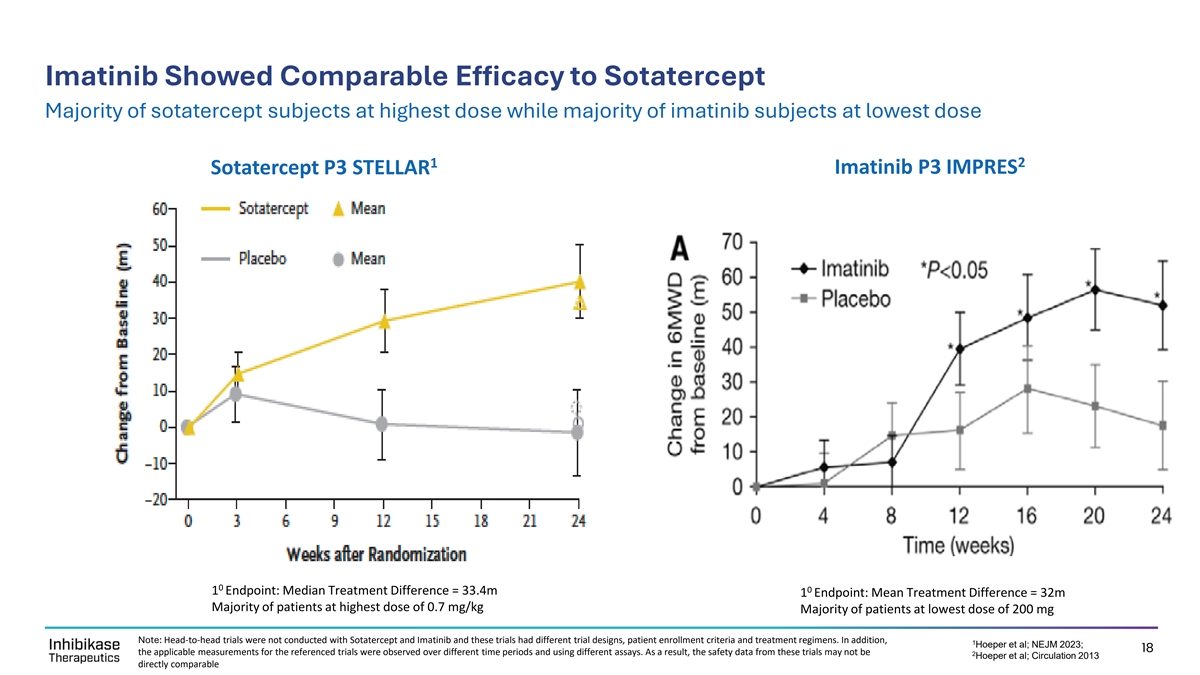
Imatinib Showed Comparable Efficacy to Sotatercept Majority of sotatercept subjects at highest dose while majority of imatinib subjects at lowest dose 1 2 Imatinib P3 IMPRES Sotatercept P3 STELLAR 0 0 1 Endpoint: Median Treatment Difference = 33.4m 1 Endpoint: Mean Treatment Difference = 32m Majority of patients at highest dose of 0.7 mg/kg Majority of patients at lowest dose of 200 mg Note: Head-to-head trials were not conducted with Sotatercept and Imatinib and these trials had different trial designs, patient enrollment criteria and treatment regimens. In addition, 1 Hoeper et al; NEJM 2023; 18 the applicable measurements for the referenced trials were observed over different time periods and using different assays. As a result, the safety data from these trials may not be 2 Hoeper et al; Circulation 2013 directly comparable

IMPRES: Patients able to sustain 400mg dose showed greater improvements Placebo-adjusted 6MWD Improvement of 45m at Week 24 in Patients at 400 mg for >50% of Treatment Changes in 6MWD at Week 24 Changes in PVR at Week 24 400mg for > 50% of 400mg for < 50% of treatment treatment Placebo 60 0 50 -10 -50 50 45 meter placebo adjusted -100 improvement in 6MWD 40 -150 -200 30 -250 22 -300 20 -350 -400 -371 10 5 -450 -437 0 -500 400mg for > 50% of 400mg for < 50% of Placebo treatment treatment N=40 N=24 N=79 N=44 N=23 N=78 -5 (Baseline*: 355 meters imatinib; 366 meters placebo) (Baseline*: 1202 dynes/sec/cm imatininb; -5 1181 dynes/sec/cm placebo) * Baseline data is not available for the sub-population of patients on 400mg of imatinib for > 50% of treatment so baseline data above reflects 19 Hoeper et al; Circulation 2013 Suppl. Appendix entire study population. PVR= Pulmonary vascular resistance; 6MWD = 6-minute walk distance Meters -5 Dynes/sec/cm
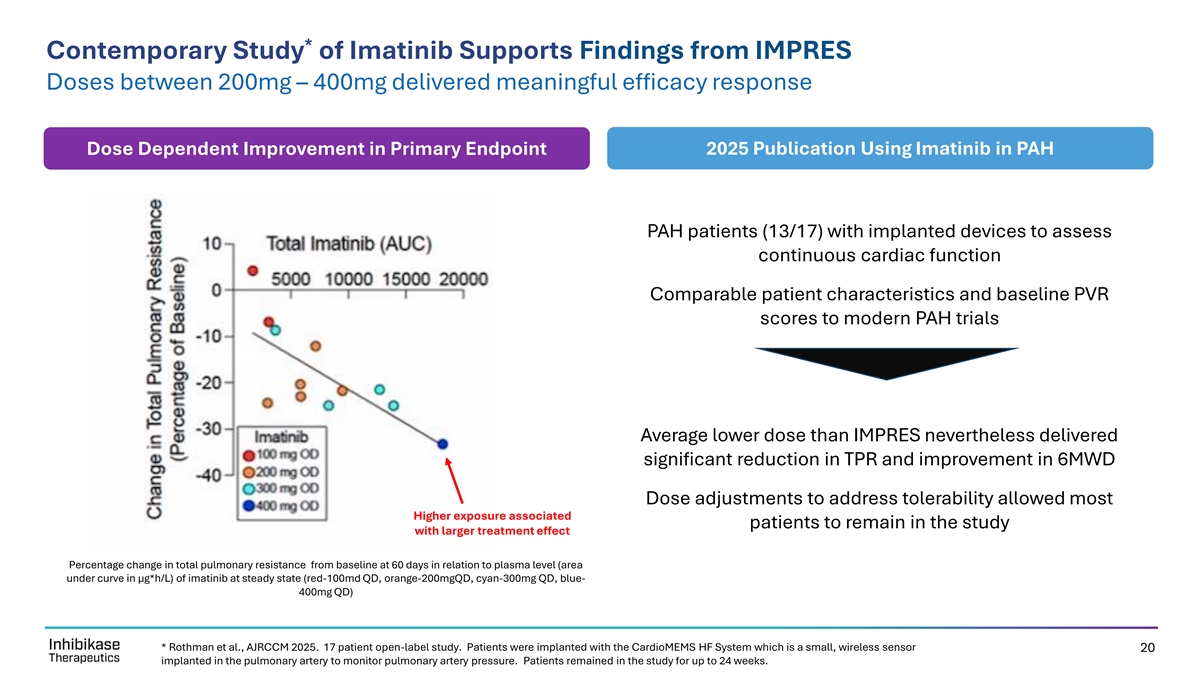
* Contemporary Study of Imatinib Supports Findings from IMPRES Doses between 200mg – 400mg delivered meaningful efficacy response Dose Dependent Improvement in Primary Endpoint 2025 Publication Using Imatinib in PAH PAH patients (13/17) with implanted devices to assess continuous cardiac function Comparable patient characteristics and baseline PVR scores to modern PAH trials Average lower dose than IMPRES nevertheless delivered significant reduction in TPR and improvement in 6MWD Dose adjustments to address tolerability allowed most Higher exposure associated patients to remain in the study with larger treatment effect Percentage change in total pulmonary resistance from baseline at 60 days in relation to plasma level (area under curve in μg*h/L) of imatinib at steady state (red-100md QD, orange-200mgQD, cyan-300mg QD, blue- 400mg QD) * Rothman et al., AJRCCM 2025. 17 patient open-label study. Patients were implanted with the CardioMEMS HF System which is a small, wireless sensor 20 implanted in the pulmonary artery to monitor pulmonary artery pressure. Patients remained in the study for up to 24 weeks.

Rapid and Sustained Hemodynamic Effect & Disease Modification of Imatinib in PAH 24% reduction in Total Pulmonary Resistance (TPR) Early & Continued Improvement Slow return to BL 1 Early Improvement 3 in TPR Slow return to baseline 2 suggests disease 24% Reduction modification in TPR * Rothman et al., AJRCCM 2025. 17 patient open-label study. Patients were implanted with the CardioMEMS HF System which is a small, wireless sensor 21 implanted in the pulmonary artery to monitor pulmonary artery pressure. Patients remained in the study for up to 24 weeks.
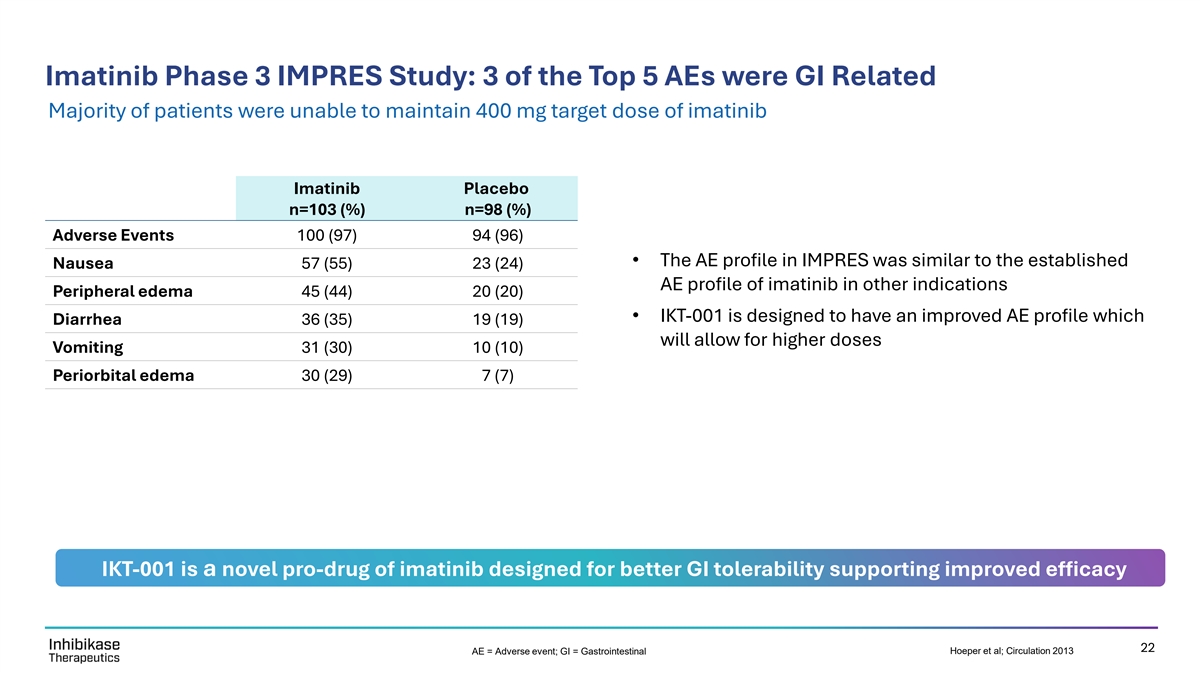
Imatinib Phase 3 IMPRES Study: 3 of the Top 5 AEs were GI Related Majority of patients were unable to maintain 400 mg target dose of imatinib Imatinib Placebo n=103 (%) n=98 (%) Adverse Events 100 (97) 94 (96) • The AE profile in IMPRES was similar to the established Nausea 57 (55) 23 (24) AE profile of imatinib in other indications Peripheral edema 45 (44) 20 (20) • IKT-001 is designed to have an improved AE profile which Diarrhea 36 (35) 19 (19) will allow for higher doses Vomiting 31 (30) 10 (10) Periorbital edema 30 (29) 7 (7) IKT-001 is a novel pro-drug of imatinib designed for better GI tolerability supporting improved efficacy 22 AE = Adverse event; GI = Gastrointestinal Hoeper et al; Circulation 2013
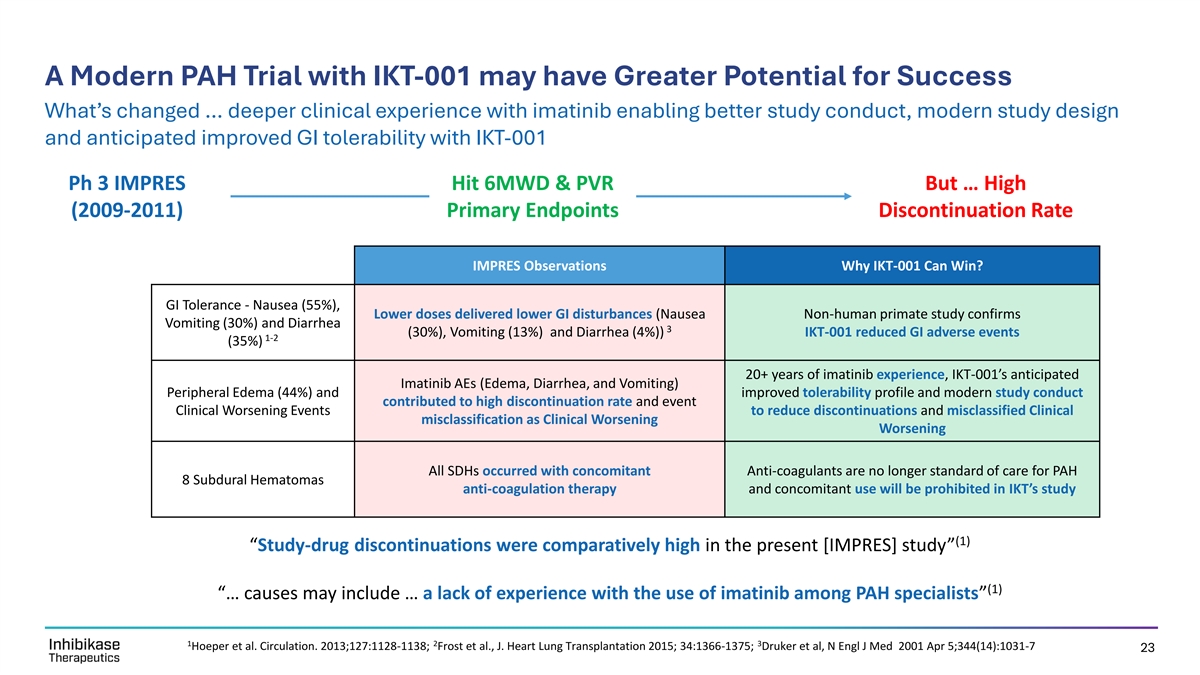
A Modern PAH Trial with IKT-001 may have Greater Potential for Success What’s changed … deeper clinical experience with imatinib enabling better study conduct, modern study design and anticipated improved GI tolerability with IKT-001 Ph 3 IMPRES Hit 6MWD & PVR But … High (2009-2011) Primary Endpoints Discontinuation Rate IMPRES Observations Why IKT-001 Can Win? GI Tolerance - Nausea (55%), Lower doses delivered lower GI disturbances (Nausea Non-human primate study confirms Vomiting (30%) and Diarrhea 3 (30%), Vomiting (13%) and Diarrhea (4%)) IKT-001 reduced GI adverse events 1-2 (35%) 20+ years of imatinib experience, IKT-001’s anticipated Imatinib AEs (Edema, Diarrhea, and Vomiting) Peripheral Edema (44%) and improved tolerability profile and modern study conduct contributed to high discontinuation rate and event Clinical Worsening Events to reduce discontinuations and misclassified Clinical misclassification as Clinical Worsening Worsening All SDHs occurred with concomitant Anti-coagulants are no longer standard of care for PAH 8 Subdural Hematomas anti-coagulation therapy and concomitant use will be prohibited in IKT’s study (1) “Study-drug discontinuations were comparatively high in the present [IMPRES] study” (1) “… causes may include … a lack of experience with the use of imatinib among PAH specialists” 1 2 3 Hoeper et al. Circulation. 2013;127:1128-1138; Frost et al., J. Heart Lung Transplantation 2015; 34:1366-1375; Druker et al, N Engl J Med 2001 Apr 5;344(14):1031-7 23

IKT’s Clinical Program
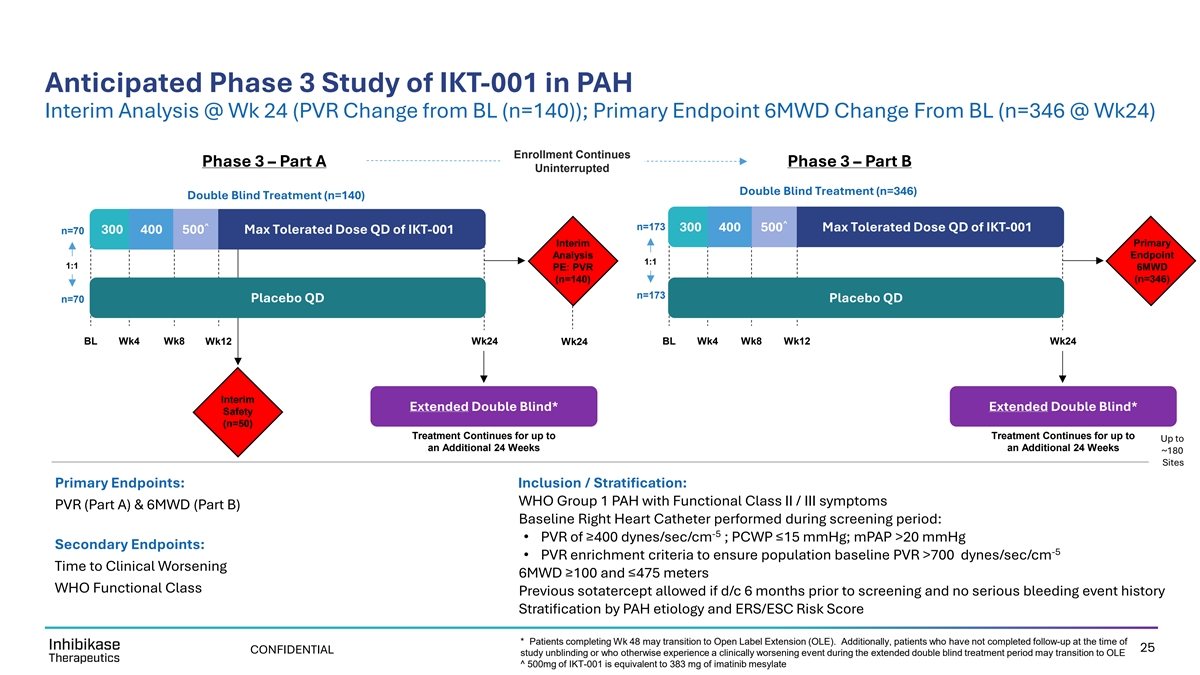
Anticipated Phase 3 Study of IKT-001 in PAH Interim Analysis @ Wk 24 (PVR Change from BL (n=140)); Primary Endpoint 6MWD Change From BL (n=346 @ Wk24) Enrollment Continues Phase 3 – Part A Phase 3 – Part B Uninterrupted Double Blind Treatment (n=346) Double Blind Treatment (n=140) ^ ^ n=173 300 400 500 Max Tolerated Dose QD of IKT-001 300 400 500 Max Tolerated Dose QD of IKT-001 n=70 Interim Primary Analysis Endpoint 1:1 1:1 PE: PVR 6MWD (n=140) (n=346) n=173 n=70 Placebo QD Placebo QD BL Wk4 Wk8 Wk12 Wk24 Wk24 BL Wk4 Wk8 Wk12 Wk24 Interim Extended Double Blind* Extended Double Blind* Safety (n=50) Treatment Continues for up to Treatment Continues for up to Up to an Additional 24 Weeks an Additional 24 Weeks ~180 Sites Primary Endpoints: Inclusion / Stratification: WHO Group 1 PAH with Functional Class II / III symptoms PVR (Part A) & 6MWD (Part B) Baseline Right Heart Catheter performed during screening period: -5 • PVR of ≥400 dynes/sec/cm ; PCWP ≤15 mmHg; mPAP >20 mmHg Secondary Endpoints: -5 • PVR enrichment criteria to ensure population baseline PVR >700 dynes/sec/cm Time to Clinical Worsening 6MWD ≥100 and ≤475 meters WHO Functional Class Previous sotatercept allowed if d/c 6 months prior to screening and no serious bleeding event history Stratification by PAH etiology and ERS/ESC Risk Score * Patients completing Wk 48 may transition to Open Label Extension (OLE). Additionally, patients who have not completed follow-up at the time of 25 CONFIDENTIAL study unblinding or who otherwise experience a clinically worsening event during the extended double blind treatment period may transition to OLE ^ 500mg of IKT-001 is equivalent to 383 mg of imatinib mesylate

Development Progress and Projected Timelines Progress Towards Initiation of IKT’s Phase 3 Study in PAH Projected Events st Expected 1 Site Activation in Phase 3 IMPROVE-PAH Study (IKT- ✓ 1H 2024 FDA Pre-IND for IKT-001 in PAH Q4 2025 / Q1 001 for Measuring Pulmonary Vascular Resistance and Outcome 2026 Variables in a Phase Evaluation of PAH ✓ 2H 2024 FDA Authority to Proceed st 1 Patient Enrolled in Part A of IMPROVE-PAH (n=140) – Primary Q1 2026 Endpoint is PVR Reduction ✓ Late 2024 $275M PIPE, IKT Pivots to PAH and New Board of Directors Mid 2026 File for Orphan Drug Designation ✓ 1H 2025 IKT Appoints New Management 1H 2027 Interim Safety Readout for Part A of IMPROVE-PAH (n=50) IKT’s Neuro Portfolio is Outlicensed and Transition to PAH Focus ✓ 1H 2025 Complete Last Patient Enrolled in Part A / First Patient Enrolled in Part B 2H 2027 (6MWD) of IMPROVE-PAH. Enrollment Expected to be Continuous ✓ Q3 2025 Phase 2b Study Design for IKT-001 in PAH + Global Site Selection Initiated Unblinded PVR Reduction Readout for Part A of IMPROVE-PAH Mid 2028 Type C Meeting Request Filed with FDA to Support Immediate Transition (n=120) ✓ Q3 2025 to Phase 3 Study End 2028 Completion of Enrollment for Part B of IMPROVE-PAH (n=346) Written Response Only (WRO) Received from FDA – Confirmed Single ✓ Q4 2025 Pivotal Study and Progression to Phase 3 is Acceptable Mid 2029 Topline Readout (6MWD) from Part B of IMPROVE-PAH (n=300) 26

Inhibikase and IKT-001: Pulmonary Arterial Hypertension (PAH) • PAH is a rare, progressive and life-threatening disease with significant unmet need Major unmet need with (1) high mortality, poor • ~30% 5-year mortality , reduced quality of life and high economic burden QoL and high cost • $7.6 Billion market with limited treatments that address the underlying etiology • Imatinib is an anti-proliferative TKI with potential best-in-class improvements in PVR and Imatinib hit efficacy (2) 6MWD (45 meters*) based on Phase 3 IMPRES and Phase 2 studies primary endpoints in Phase 3 in PAH • Imatinib was not approved due to high discontinuation rate in Phase 3 IMPRES st • 2 decades of imatinib clinical experience, together with IKT-001’s improved GI tolerability profile Potential to be the 1 and better-informed study design / study conduct supports potential higher probability of success oral anti-proliferative agent • IKT’s pro-drug is engineered to realize the potential of imatinib in PAH & lower discontinuations • IKT’s Phase 3 is on track to initiate in Q1 of 2026 Strong Leadership • Long intellectual property (NCE) runway through 2044 Executing Near Term • Proven executive team with extensive PAH / CV experience Development (1) Hoeper M, et al. Eur Respir J. 2017 (2) Studies conducted by Novartis Pharmaceutical Corporation 27 TKI = tyrosine kinase inhibitor; QoL= Quality of Life; PVR = pulmonary vascular resistance; 6MWD = 6-minute walk distance; GI= Gastrointestinal; CV = cardiovascular * See Page 19

Thank You