

Exhibit 99.1 March 22, 2023| Inhibikase Neurodegeneration R&D Day Transforming fundamental and clinical research in neurodegenerative disease Inhibikase.com : IKT

DISCLAIMER This presentation shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. This presentation contains information that may constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended. Inhibikase Therapeutics, Inc. (the “Company” or “we”) intends for the forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in those sections. Generally, we have identified such forward-looking statements by using the words “believe,” “expect,” “intend,” “estimate,” “anticipate,” “project,” “target,” “forecast,” “aim,” should, “will,” may”, “continue” and similar expressions. Such statements are subject to a number of assumptions, risks and uncertainties which may cause actual results, performance or achievements to be materially different from those anticipated in these forward-looking statements. You should read statements that contain these words carefully because they discuss future expectations and plans which contain projections of future clinical studies, regulatory approvals, product candidate development, results of operations or financial condition or state other forward-looking information. However, the absence of these words or similar expressions does not mean that a statement is not forward-looking. Forward-looking statements are not historical facts, but instead represent only the Company’s beliefs regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company’s control. It is possible that the Company’s actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Management believes that these forward-looking statements are reasonable as of the time made. However, caution should be taken not to place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from the Company's historical experience and our present expectations or projections. Important factors that could cause actual results to differ materially from those in the forward-looking statements are set forth in the Company’s filings with the Securities and Exchange Commission, including its annual report on Form 10-K and its quarterly Form 10-Q, including under the caption Risk Factors”. We do not intend our use or display of other entities’ names, trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity. Inhibikase.com • : IKT 2

Agenda for the 2023 Neurodegeneration R&D Day • Why is c-Abl an important target in PD? • How was IkT-148009 discovered? • What is therapeutically possible with IkT-148009? • Clinical Development: Phase 2 and beyond in PD • Opening of MSA clinical development In Inh hiib biik ka as se e.c .co om m • • :: IK IKT T 3 3
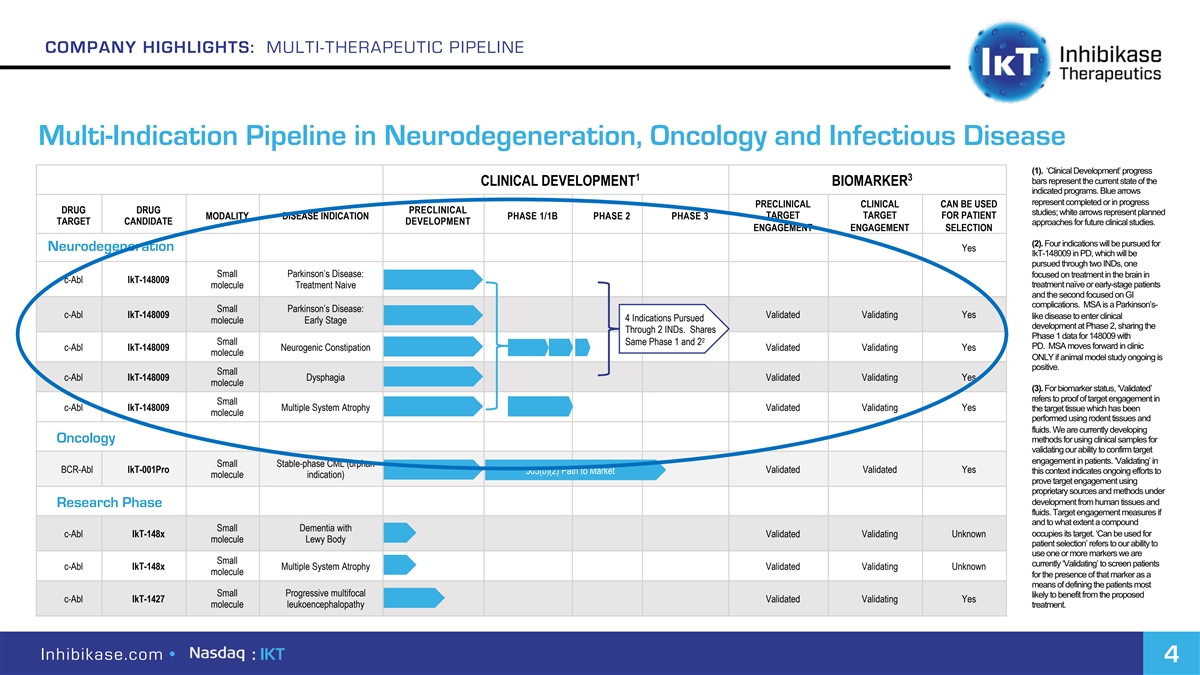
COMPANY HIGHLIGHTS: MULTI-THERAPEUTIC PIPELINE Multi-Indication Pipeline in Neurodegeneration, Oncology and Infectious Disease (1). ‘Clinical Development’ progress 1 3 bars represent the current state of the CLINICAL DEVELOPMENT BIOMARKER indicated programs. Blue arrows represent completed or in progress PRECLINICAL CLINICAL CAN BE USED DRUG DRUG PRECLINICAL studies; white arrows represent planned TARGET TARGET FOR PATIENT MODALITY DISEASE INDICATION PHASE 1/1B PHASE 2 PHASE 3 TARGET CANDIDATE DEVELOPMENT approaches for future clinical studies. ENGAGEMENT ENGAGEMENT SELECTION (2). Four indications will be pursued for Neurodegeneration Yes IkT-148009 in PD, which will be pursued through two INDs, one Small Parkinson’s Disease: focused on treatment in the brain in c-Abl IkT-148009 treatment naïve or early-stage patients molecule Treatment Naive and the second focused on GI complications. MSA is a Parkinson’s- Small Parkinson’s Disease: c-Abl IkT-148009 Validated Validating Yes like disease to enter clinical 4 Indications Pursued molecule Early Stage development at Phase 2, sharing the Through 2 INDs. Shares Phase 1 data for 148009 with 2 Small Same Phase 1 and 2 PD. MSA moves forward in clinic c-Abl IkT-148009 Neurogenic Constipation Validated Validating Yes molecule ONLY if animal model study ongoing is positive. Small c-Abl IkT-148009 Dysphagia Validated Validating Yes molecule (3). For biomarker status, ‘Validated’ refers to proof of target engagement in Small c-Abl IkT-148009 Multiple System Atrophy Validated Validating Yes the target tissue which has been molecule performed using rodent tissues and fluids. We are currently developing Oncology methods for using clinical samples for validating our ability to confirm target engagement in patients. ‘Validating’ in Small Stable-phase CML (orphan BCR-Abl IkT-001Pro 505(b)(2) Path to Market Validated Validated Yes this context indicates ongoing efforts to molecule indication) prove target engagement using proprietary sources and methods under development from human tissues and Research Phase fluids. Target engagement measures if and to what extent a compound Small Dementia with occupies its target. ‘Can be used for c-Abl IkT-148x Validated Validating Unknown molecule Lewy Body patient selection’ refers to our ability to use one or more markers we are Small currently ‘Validating’ to screen patients c-Abl IkT-148x Multiple System Atrophy Validated Validating Unknown molecule for the presence of that marker as a means of defining the patients most Small Progressive multifocal likely to benefit from the proposed c-Abl IkT-1427 Validated Validating Yes molecule leukoencephalopathy treatment. Inhibikase.com • : IKT 4
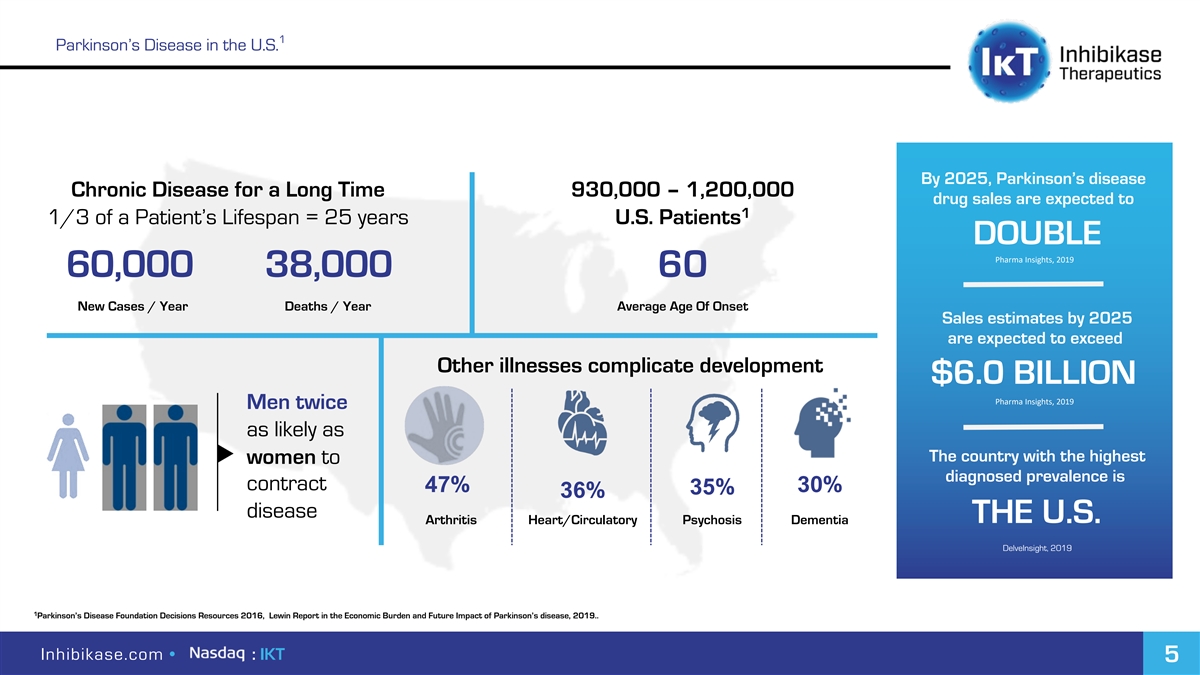
1 Parkinson’s Disease in the U.S. By 2025, Parkinson’s disease Chronic Disease for a Long Time 930,000 – 1,200,000 drug sales are expected to 1 1/3 of a Patient’s Lifespan = 25 years U.S. Patients DOUBLE Pharma Insights, 2019 60,000 38,000 60 New Cases / Year Deaths / Year Average Age Of Onset Sales estimates by 2025 are expected to exceed Other illnesses complicate development $6.0 BILLION Pharma Insights, 2019 Men twice as likely as The country with the highest women to diagnosed prevalence is contract 47% 30% 35% 36% disease THE U.S. Arthritis Heart/Circulatory Psychosis Dementia DelveInsight, 2019 1 Parkinson’s Disease Foundation Decisions Resources 2016, Lewin Report in the Economic Burden and Future Impact of Parkinson’s disease, 2019.. Inhibikase.com • : IKT 5

Discovery of c-Abl’s Role in PD In Inh hiib biik ka as se e.c .co om m • • :: IK IKT T 6 6
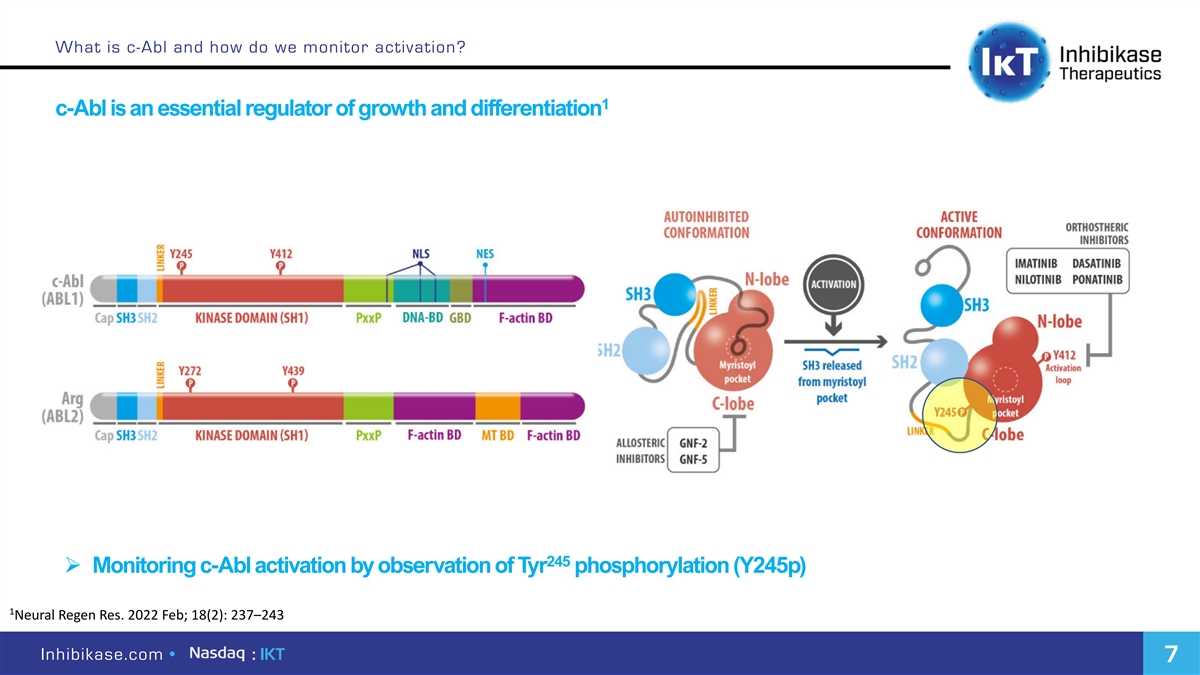
What is c-Abl and how do we monitor activation? 1 c-Abl is an essential regulator of growth and differentiation 245 Ø Monitoring c-Abl activation by observation of Tyr phosphorylation (Y245p) 1 Neural Regen Res. 2022 Feb; 18(2): 237–243 Inhibikase.com • : IKT 7
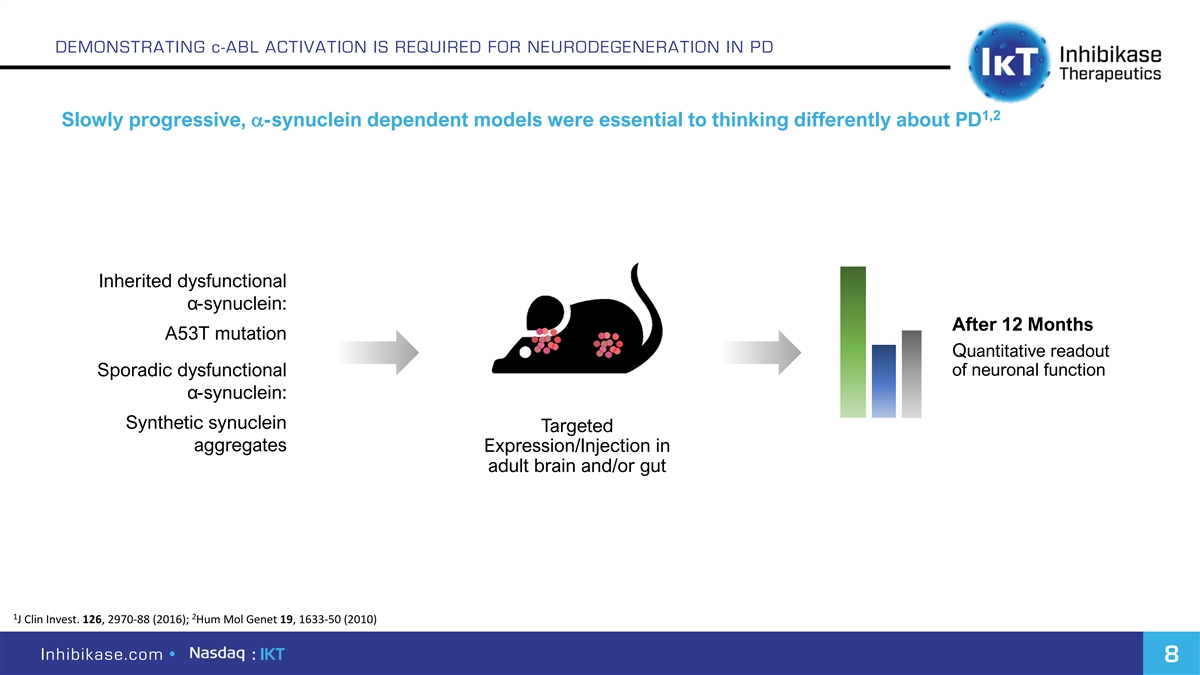
DEMONSTRATING c-ABL ACTIVATION IS REQUIRED FOR NEURODEGENERATION IN PD 1,2 Slowly progressive, a-synuclein dependent models were essential to thinking differently about PD Inherited dysfunctional α-synuclein: After 12 Months A53T mutation Quantitative readout Sporadic dysfunctional of neuronal function α-synuclein: Synthetic synuclein Targeted aggregates Expression/Injection in adult brain and/or gut 1 2 J Clin Invest. 126, 2970-88 (2016); Hum Mol Genet 19, 1633-50 (2010) Inhibikase.com • : IKT 8
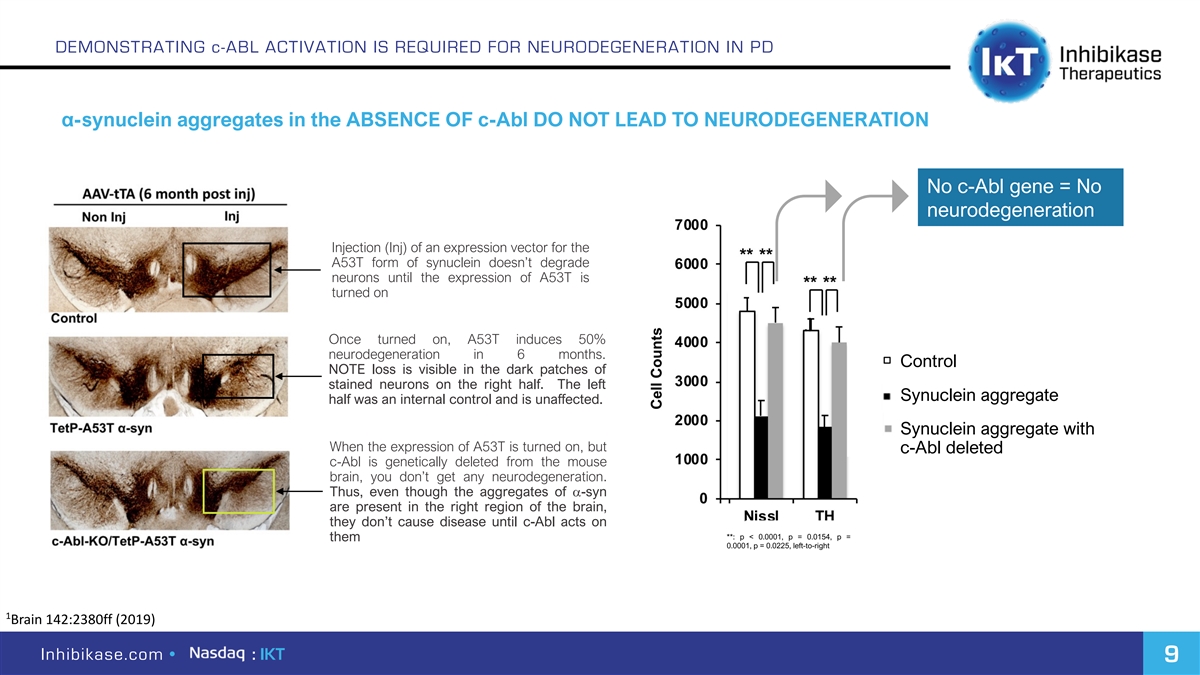
DEMONSTRATING c-ABL ACTIVATION IS REQUIRED FOR NEURODEGENERATION IN PD α-synuclein aggregates in the ABSENCE OF c-Abl DO NOT LEAD TO NEURODEGENERATION No c-Abl gene = No neurodegeneration Injection (Inj) of an expression vector for the A53T form of synuclein doesn’t degrade neurons until the expression of A53T is turned on Once turned on, A53T induces 50% neurodegeneration in 6 months. Control NOTE loss is visible in the dark patches of stained neurons on the right half. The left Synuclein aggregate half was an internal control and is unaffected. Synuclein aggregate with When the expression of A53T is turned on, but c-Abl deleted c-Abl is genetically deleted from the mouse brain, you don’t get any neurodegeneration. Thus, even though the aggregates of a-syn are present in the right region of the brain, they don’t cause disease until c-Abl acts on **: p < 0.0001, p = 0.0154, p = them 0.0001, p = 0.0225, left-to-right 1 Brain 142:2380ff (2019) Inhibikase.com • : IKT 9
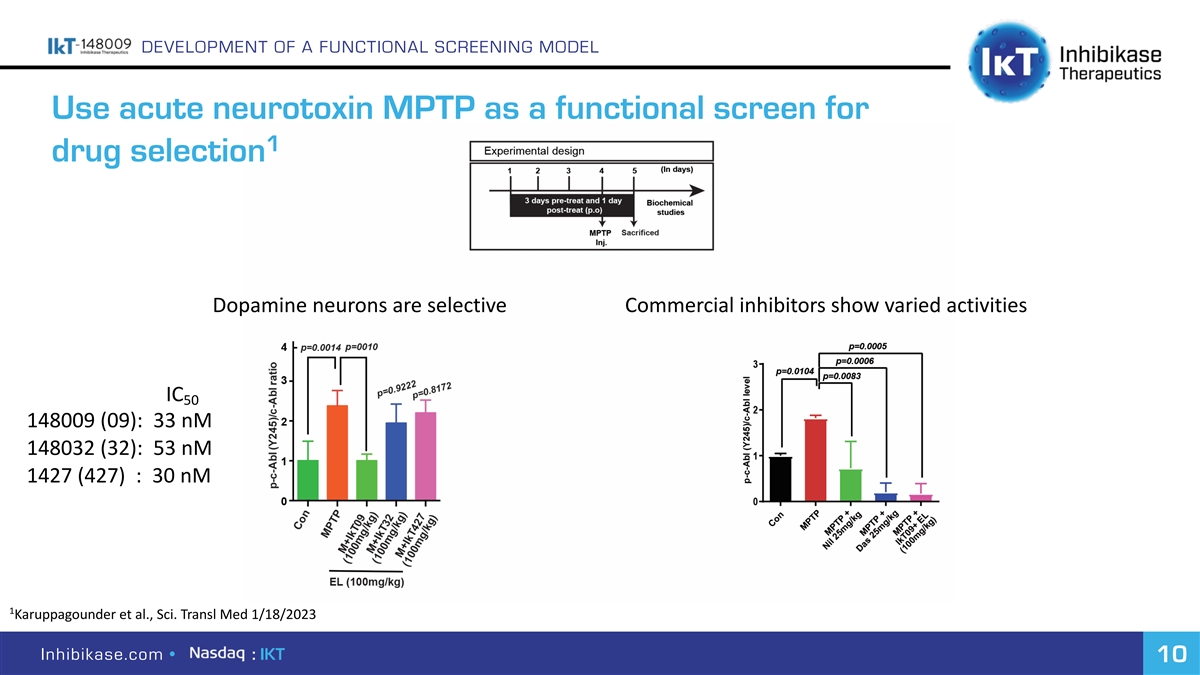
DEVELOPMENT OF A FUNCTIONAL SCREENING MODEL Use acute neurotoxin MPTP as a functional screen for 1 drug selection Dopamine neurons are selective Commercial inhibitors show varied activities IC 50 148009 (09): 33 nM 148032 (32): 53 nM 1427 (427) : 30 nM 1 Karuppagounder et al., Sci. Transl Med 1/18/2023 Inhibikase.com • : IKT 10

DEMONSTRATING THE POTENTIAL FOR c-Abl INHIBITION TO PROTECT NEURONS IN THE BRAIN 1 Pre-treatment with IkT-148009 blocks neurodegeneration in the MPTP acute toxicity model No IkT-148009 Pre-Treatment with IkT-148009 Mice treated with an acute neurotoxin MPTP Mice pre-treated with IkT-148009 are protected results in substantial loss of dopamine neurons from neurodegeneration Ø No stimulus response, hunched Ø Normal response to stimuli Ø Little movement Ø Run around the cage Ø Anti-social Ø Socially engaged 1 Karuppagounder et al., Sci. Transl Med 1/18/2023 Inhibikase.com • : IKT 11
Initial conclusions What we concluded early on about c-Abl inhibition as a potential 1 disease-modifying therapy for PD? Ø C-Abl is an essential determinant for PD initiation and progression: If you delete the gene, you cannot induce neurodegeneration in animals Ø C-Abl inhibition can block induction of neurodegeneration by the acute neurotoxin MPTP 1 Werner and Olanow, Mov. Disord. 2022 DOI: 10.1002/mds.28858 Inhibikase.com • : IKT 12
Discovery of IkT-148009 and its potential therapeutic benefit in PD In Inh hiib biik ka as se e.c .co om m • • :: IK IKT T 13 13
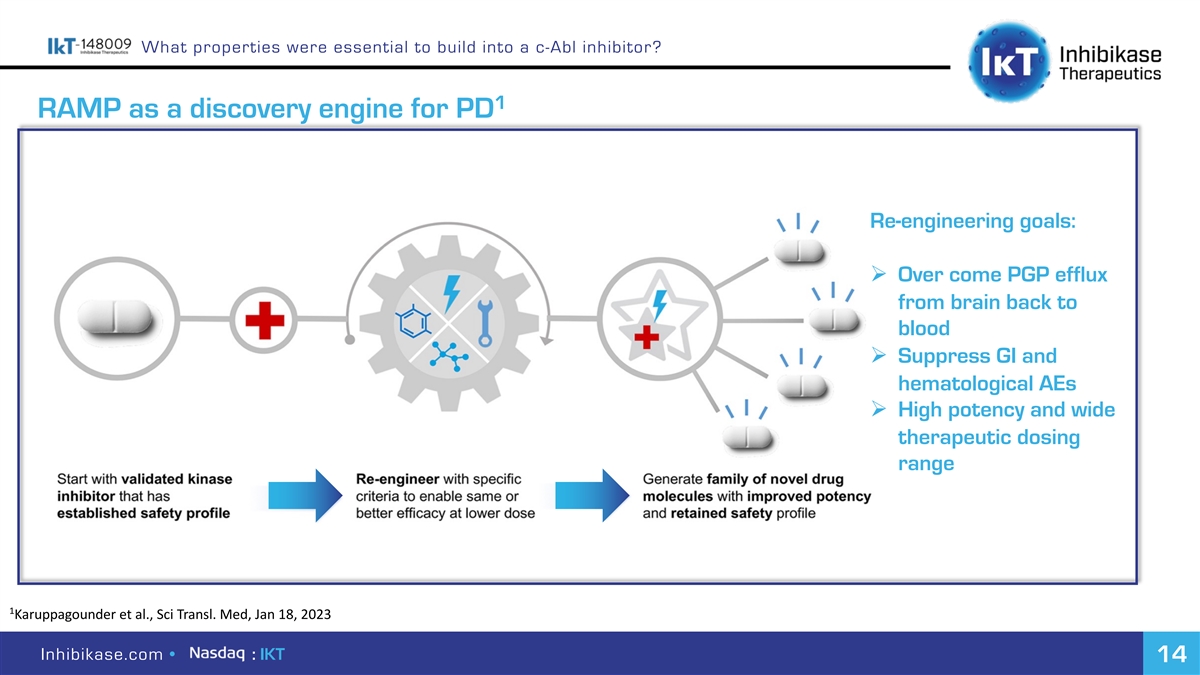
What properties were essential to build into a c-Abl inhibitor? 1 RAMP as a discovery engine for PD Re-engineering goals: Ø Over come PGP efflux from brain back to blood Ø Suppress GI and hematological AEs Ø High potency and wide therapeutic dosing range 1 Karuppagounder et al., Sci Transl. Med, Jan 18, 2023 Inhibikase.com • : IKT 14
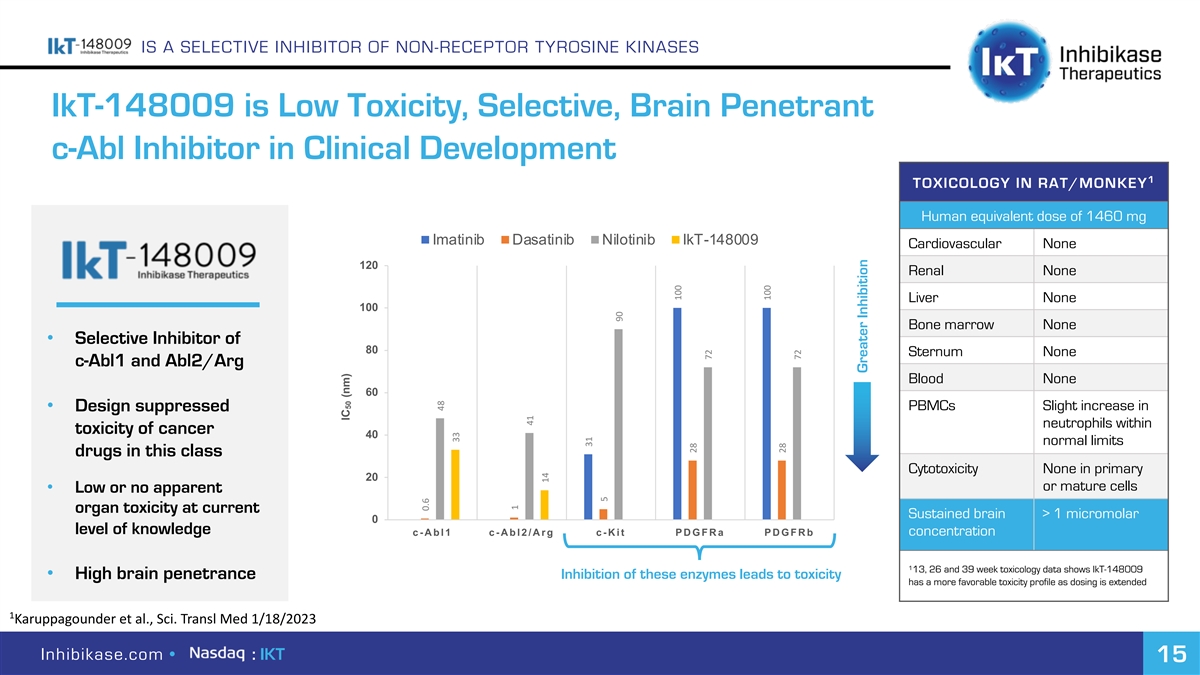
IS A SELECTIVE INHIBITOR OF NON-RECEPTOR TYROSINE KINASES IkT-148009 is Low Toxicity, Selective, Brain Penetrant c-Abl Inhibitor in Clinical Development 1 TOXICOLOGY IN RAT/MONKEY Human equivalent dose of 1460 mg Imatinib Dasatinib Nilotinib IkT-148009 Cardiovascular None 120 Renal None Liver None 100 Bone marrow None • Selective Inhibitor of 80 Sternum None c-Abl1 and Abl2/Arg Blood None 60 PBMCs Slight increase in • Design suppressed neutrophils within toxicity of cancer 40 normal limits drugs in this class Cytotoxicity None in primary 20 or mature cells • Low or no apparent organ toxicity at current Sustained brain > 1 micromolar 0 level of knowledge c-Abl1 c-Abl2/Arg c-Kit PDGFRa PDGFRb concentration 1 13, 26 and 39 week toxicology data shows IkT-148009 • High brain penetrance Inhibition of these enzymes leads to toxicity has a more favorable toxicity profile as dosing is extended 1 Karuppagounder et al., Sci. Transl Med 1/18/2023 Inhibikase.com • : IKT 15 IC (nm) 50 0.6 48 33 1 41 14 31 5 90 100 28 72 100 28 72 Greater Inhibition
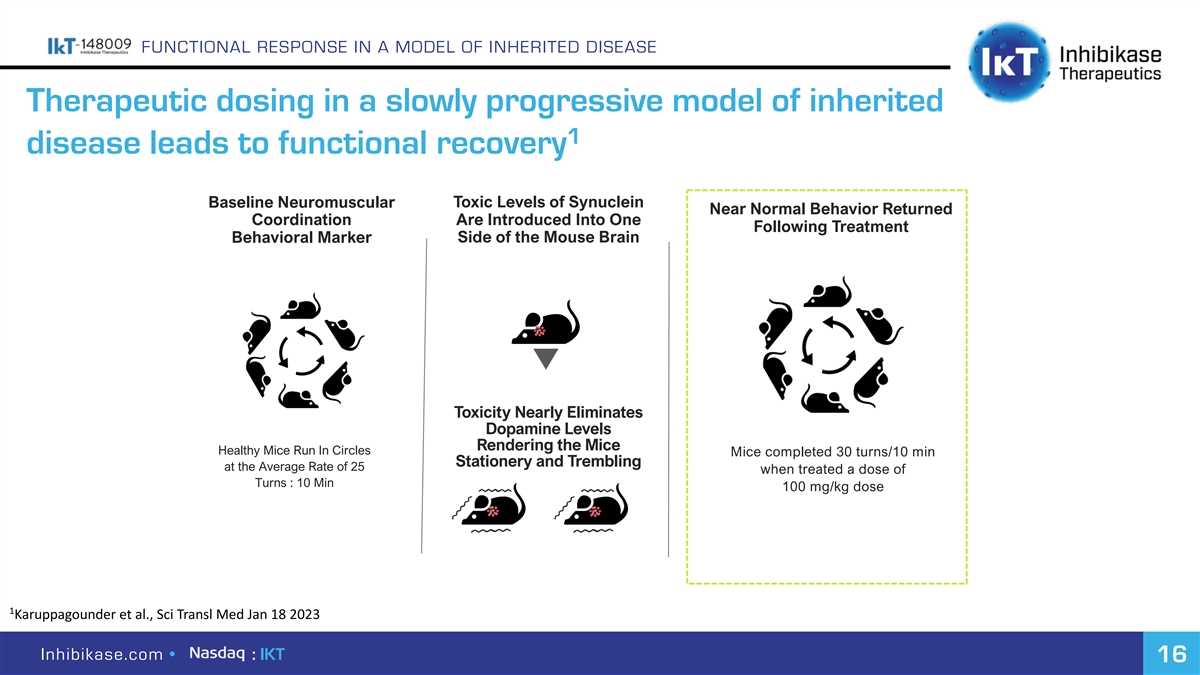
FUNCTIONAL RESPONSE IN A MODEL OF INHERITED DISEASE Therapeutic dosing in a slowly progressive model of inherited 1 disease leads to functional recovery Baseline Neuromuscular Toxic Levels of Synuclein Near Normal Behavior Returned Coordination Are Introduced Into One Following Treatment Behavioral Marker Side of the Mouse Brain Toxicity Nearly Eliminates Dopamine Levels Rendering the Mice Healthy Mice Run In Circles Mice completed 30 turns/10 min Stationery and Trembling at the Average Rate of 25 when treated a dose of Turns : 10 Min 100 mg/kg dose 1 Karuppagounder et al., Sci Transl Med Jan 18 2023 Inhibikase.com • : IKT 16
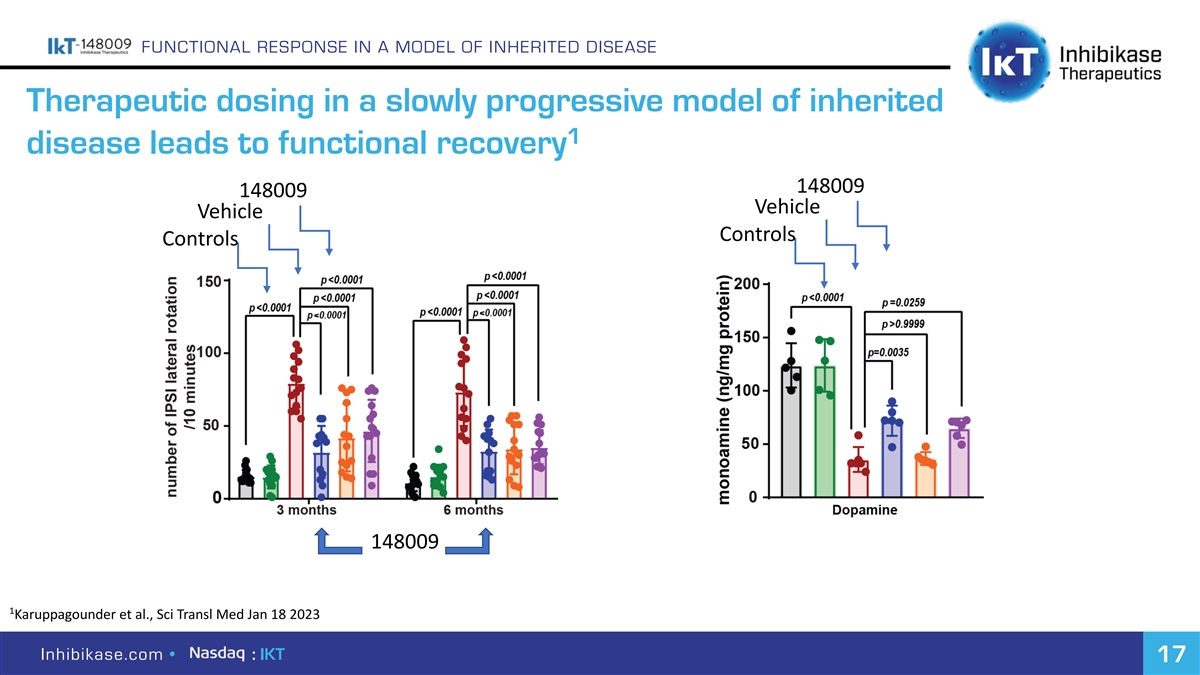
FUNCTIONAL RESPONSE IN A MODEL OF INHERITED DISEASE Therapeutic dosing in a slowly progressive model of inherited 1 disease leads to functional recovery 148009 148009 Vehicle Vehicle Controls Controls 148009 1 Karuppagounder et al., Sci Transl Med Jan 18 2023 Inhibikase.com • : IKT 17
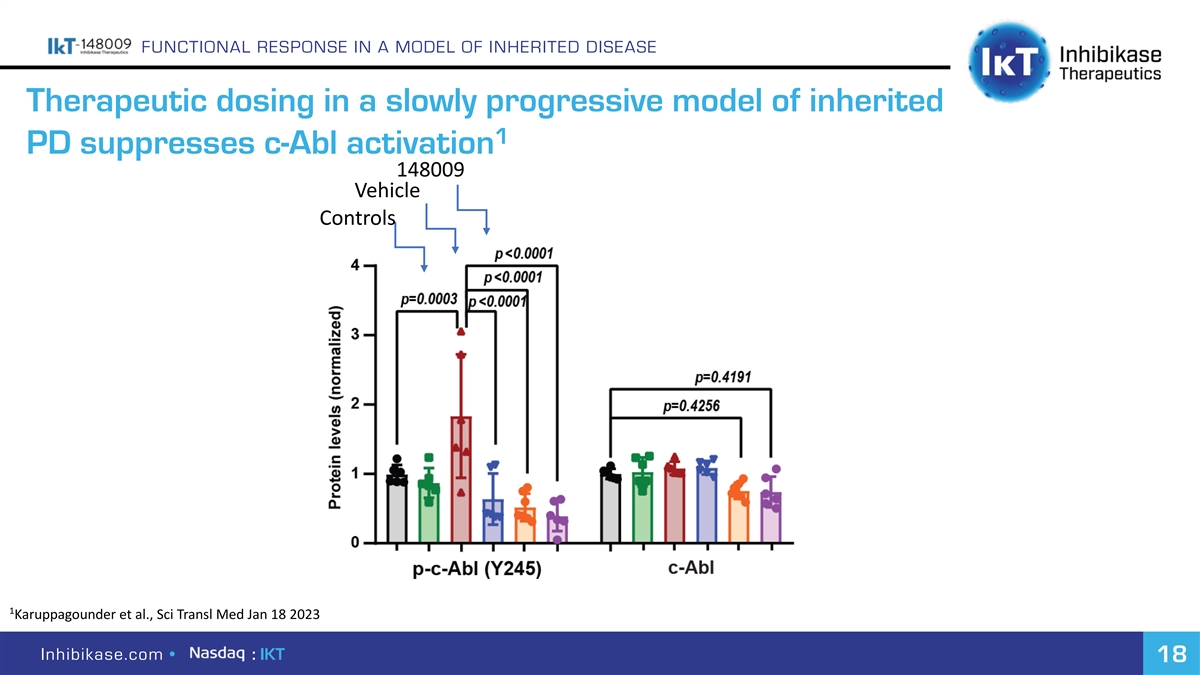
FUNCTIONAL RESPONSE IN A MODEL OF INHERITED DISEASE Therapeutic dosing in a slowly progressive model of inherited 1 PD suppresses c-Abl activation 148009 Vehicle Controls 1 Karuppagounder et al., Sci Transl Med Jan 18 2023 Inhibikase.com • : IKT 18
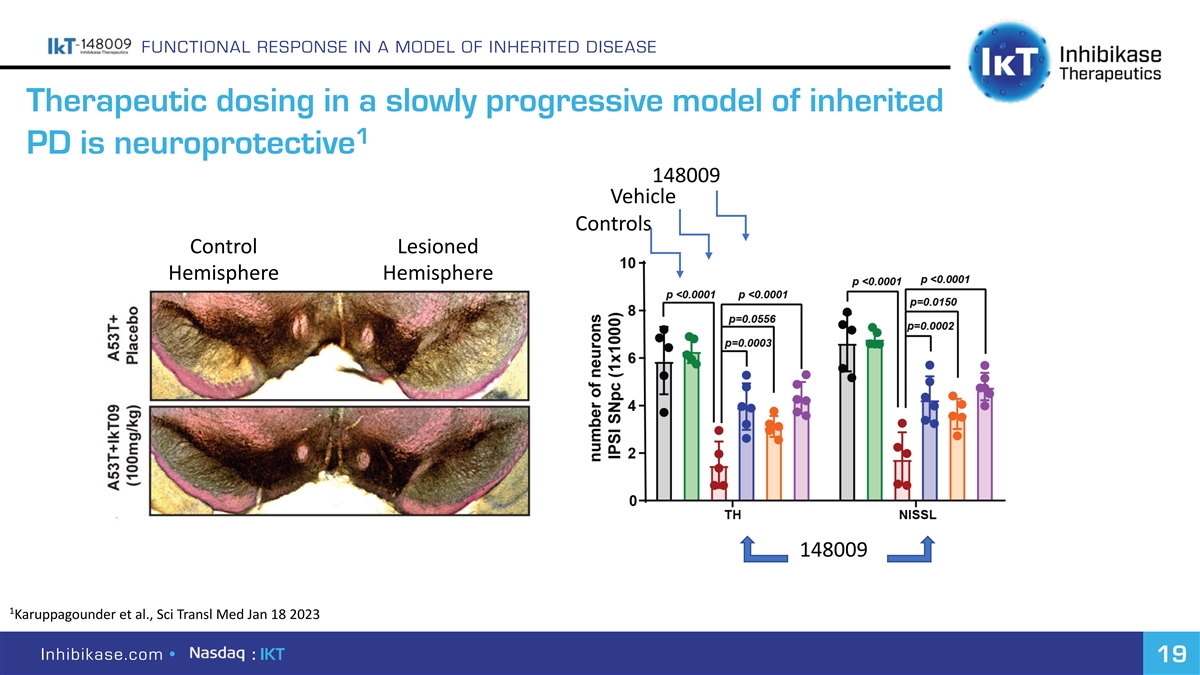
FUNCTIONAL RESPONSE IN A MODEL OF INHERITED DISEASE Therapeutic dosing in a slowly progressive model of inherited 1 PD is neuroprotective 148009 Vehicle Controls Control Lesioned Hemisphere Hemisphere 148009 1 Karuppagounder et al., Sci Transl Med Jan 18 2023 Inhibikase.com • : IKT 19
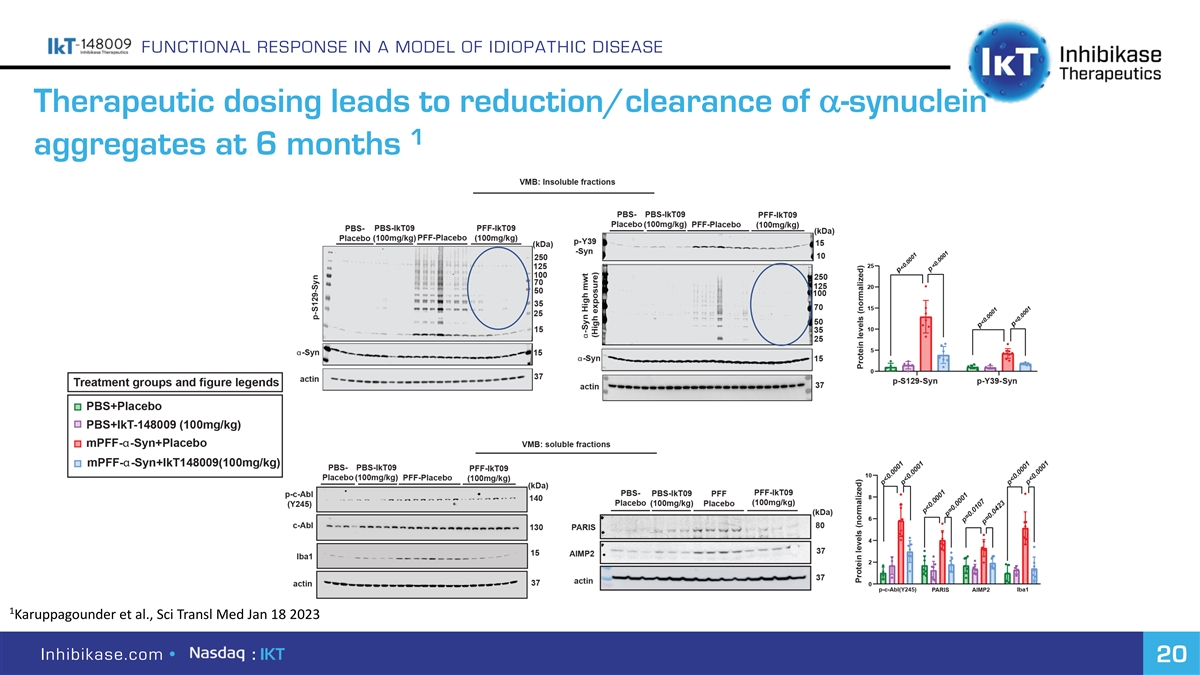
FUNCTIONAL RESPONSE IN A MODEL OF IDIOPATHIC DISEASE a Therapeutic dosing leads to reduction/clearance of -synuclein 1 aggregates at 6 months 1 Karuppagounder et al., Sci Transl Med Jan 18 2023 Inhibikase.com • : IKT 20

THERAPEUTIC BENEFIT OF ONCE DAILY ORAL IkT-148009 IN ANIMAL MODELS Modeling PD followed by therapeutic dosing revealed: Ø C-Abl inhibition is neuroprotective in animal models of disease Ø C-Abl inhibition blocks downstream effector pathways of neurodegeneration in animal models of disease Ø C-Abl inhibition reduces a-synuclein pathology in the affected neurons in animal models of disease Inhibikase.com • : IKT 21
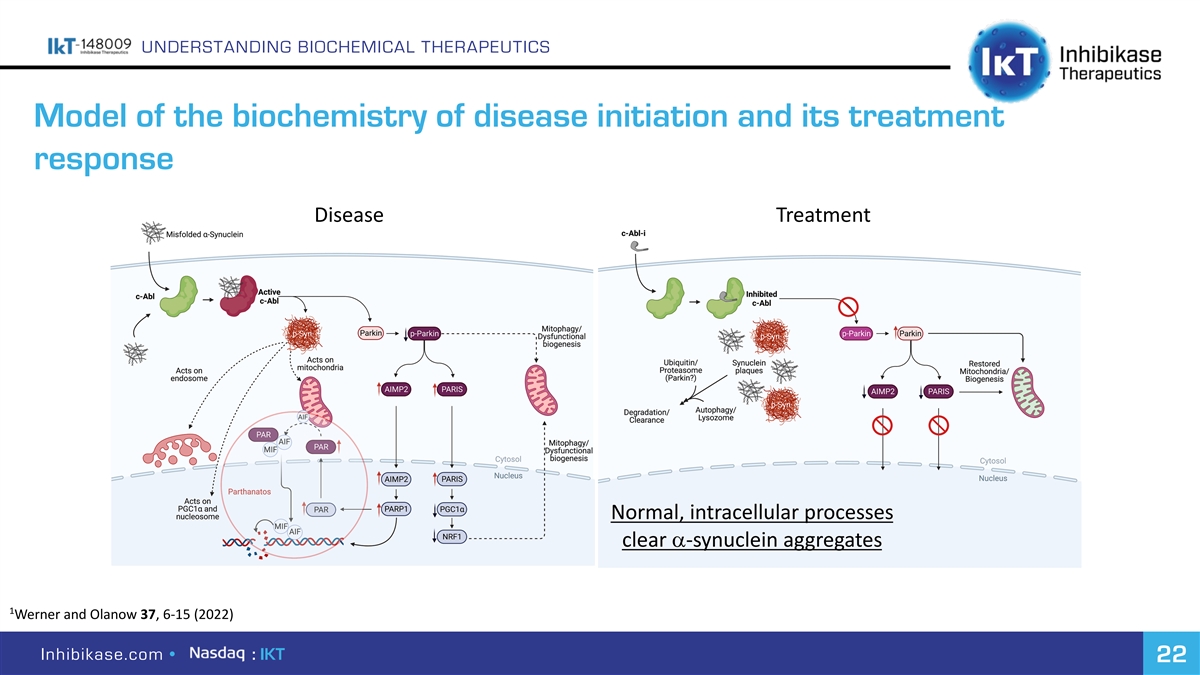
UNDERSTANDING BIOCHEMICAL THERAPEUTICS Model of the biochemistry of disease initiation and its treatment response Disease Treatment Normal, intracellular processes clear a-synuclein aggregates 1 Werner and Olanow37, 6-15 (2022) Inhibikase.com • : IKT 22
Clinical Development of IkT-148009 for Treatment of Parkinson’s disease Inhibikase.com • : IKT 23
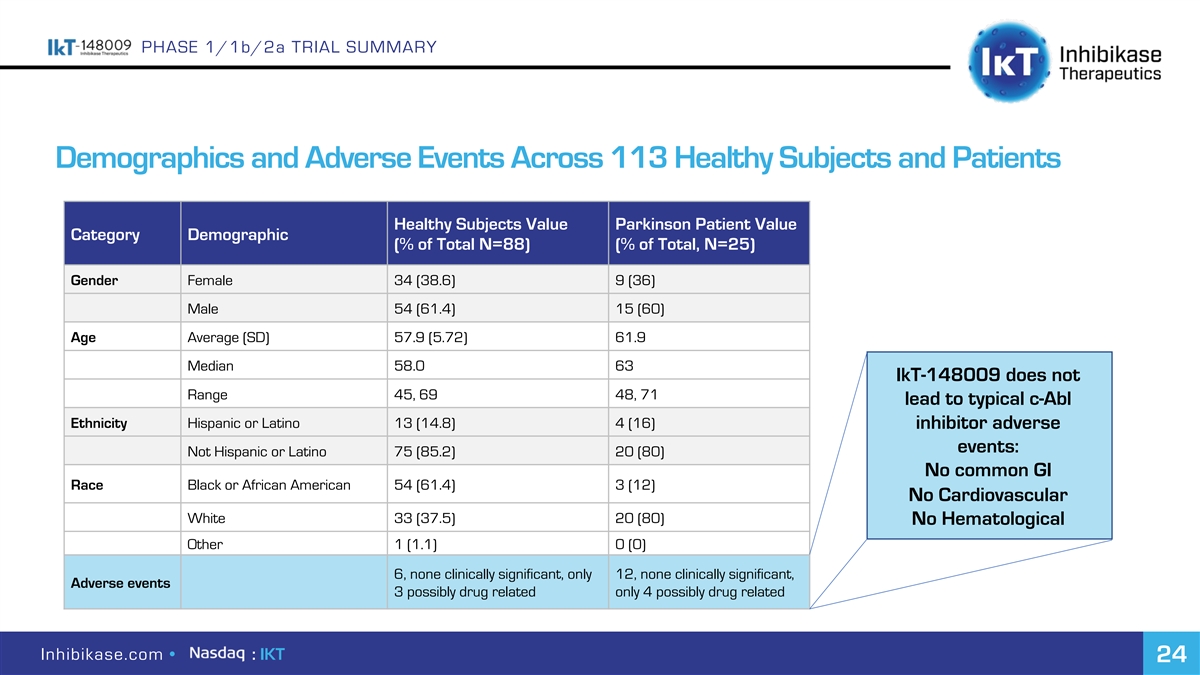
PHASE 1/1b/2a TRIAL SUMMARY Demographics and Adverse Events Across 113 Healthy Subjects and Patients Healthy Subjects Value Parkinson Patient Value Category Demographic (% of Total N=88) (% of Total, N=25) Gender Female 34 (38.6) 9 (36) Male 54 (61.4) 15 (60) Age Average (SD) 57.9 (5.72) 61.9 Median 58.0 63 IkT-148009 does not Range 45, 69 48, 71 lead to typical c-Abl Ethnicity Hispanic or Latino 13 (14.8) 4 (16) inhibitor adverse events: Not Hispanic or Latino 75 (85.2) 20 (80) No common GI Race Black or African American 54 (61.4) 3 (12) No Cardiovascular White 33 (37.5) 20 (80) No Hematological Other 1 (1.1) 0 (0) 6, none clinically significant, only 12, none clinically significant, Adverse events 3 possibly drug related only 4 possibly drug related Inhibikase.com • : IKT 24
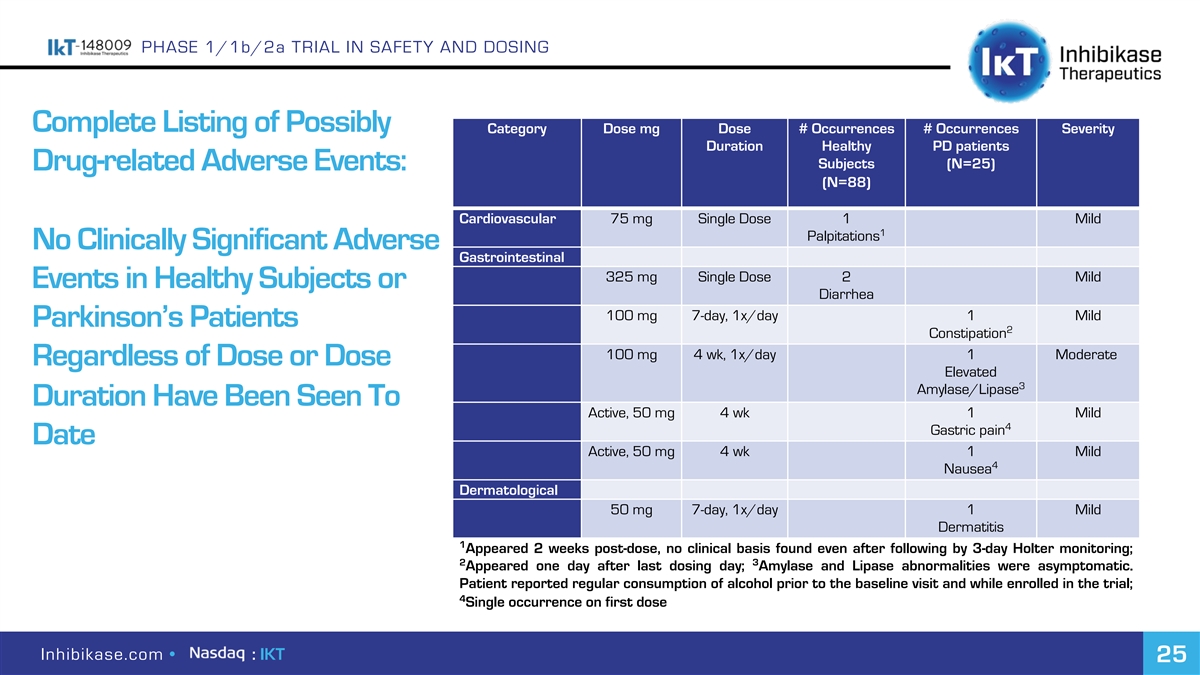
PHASE 1/1b/2a TRIAL IN SAFETY AND DOSING Complete Listing of Possibly Category Dose mg Dose # Occurrences # Occurrences Severity Duration Healthy PD patients Subjects (N=25) Drug-related Adverse Events: (N=88) Cardiovascular 75 mg Single Dose 1 Mild 1 Palpitations No Clinically Significant Adverse Gastrointestinal 325 mg Single Dose 2 Mild Events in Healthy Subjects or Diarrhea 100 mg 7-day, 1x/day 1 Mild Parkinson’s Patients 2 Constipation 100 mg 4 wk, 1x/day 1 Moderate Regardless of Dose or Dose Elevated 3 Amylase/Lipase Duration Have Been Seen To Active, 50 mg 4 wk 1 Mild 4 Gastric pain Date Active, 50 mg 4 wk 1 Mild 4 Nausea Dermatological 50 mg 7-day, 1x/day 1 Mild Dermatitis 1 Appeared 2 weeks post-dose, no clinical basis found even after following by 3-day Holter monitoring; 2 3 Appeared one day after last dosing day; Amylase and Lipase abnormalities were asymptomatic. Patient reported regular consumption of alcohol prior to the baseline visit and while enrolled in the trial; 4 Single occurrence on first dose Inhibikase.com • : IKT 25
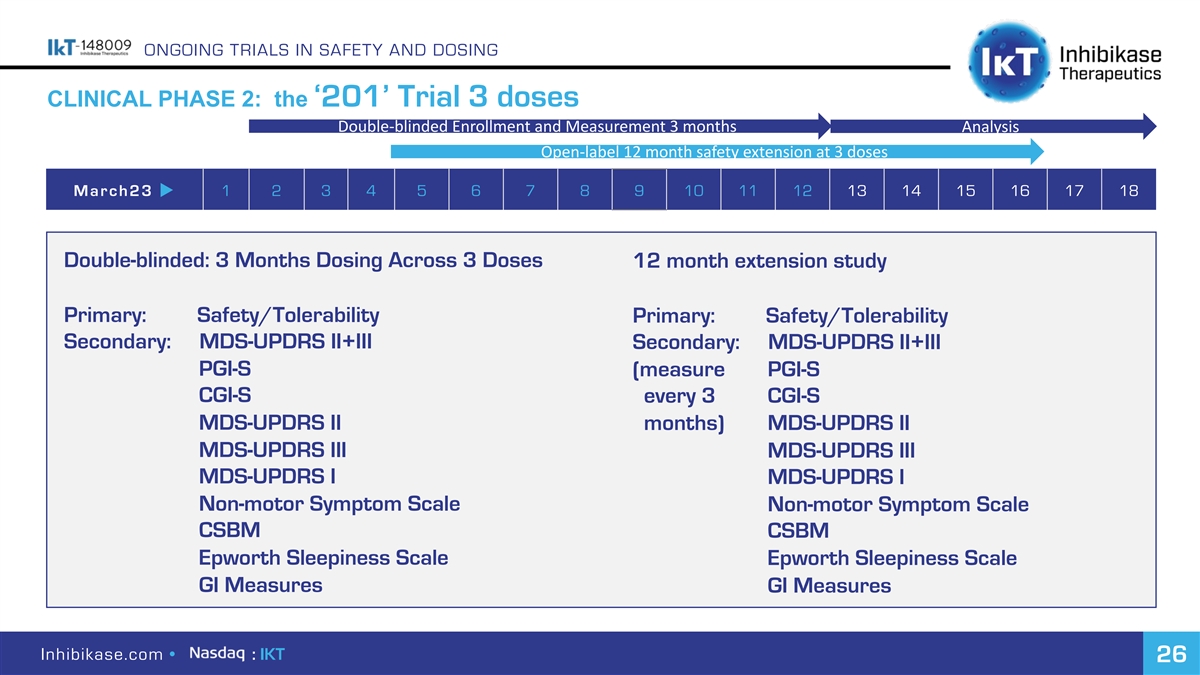
ONGOING TRIALS IN SAFETY AND DOSING CLINICAL PHASE 2: the ‘201’ Trial 3 doses Double-blinded Enrollment and Measurement 3 months Analysis Open-label 12 month safety extension at 3 doses March23 u 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Double-blinded: 3 Months Dosing Across 3 Doses 12 month extension study Primary: Safety/Tolerability Primary: Safety/Tolerability Secondary: MDS-UPDRS II+III Secondary: MDS-UPDRS II+III PGI-S (measure PGI-S CGI-S every 3 CGI-S MDS-UPDRS II months) MDS-UPDRS II MDS-UPDRS III MDS-UPDRS III MDS-UPDRS I MDS-UPDRS I Non-motor Symptom Scale Non-motor Symptom Scale CSBM CSBM Epworth Sleepiness Scale Epworth Sleepiness Scale GI Measures GI Measures Inhibikase.com • : IKT 26
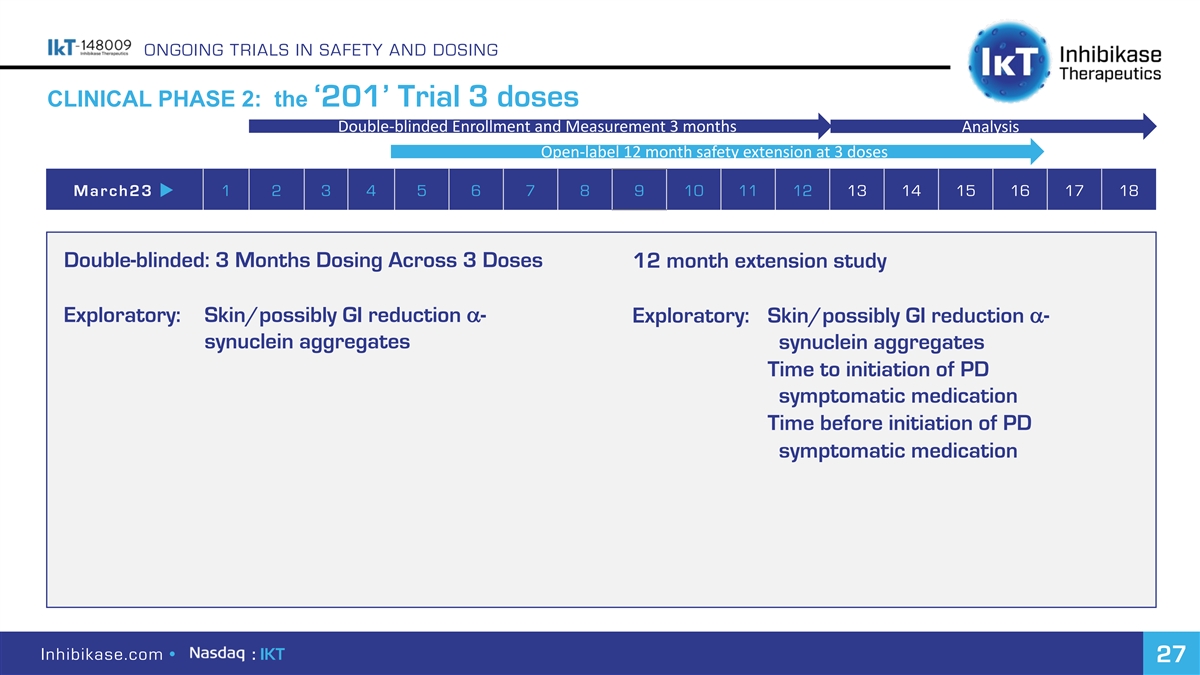
ONGOING TRIALS IN SAFETY AND DOSING CLINICAL PHASE 2: the ‘201’ Trial 3 doses Double-blinded Enrollment and Measurement 3 months Analysis Open-label 12 month safety extension at 3 doses March23 u 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Double-blinded: 3 Months Dosing Across 3 Doses 12 month extension study Exploratory: Skin/possibly GI reduction a- Exploratory: Skin/possibly GI reduction a- synuclein aggregates synuclein aggregates Time to initiation of PD symptomatic medication Time before initiation of PD symptomatic medication Inhibikase.com • : IKT 27
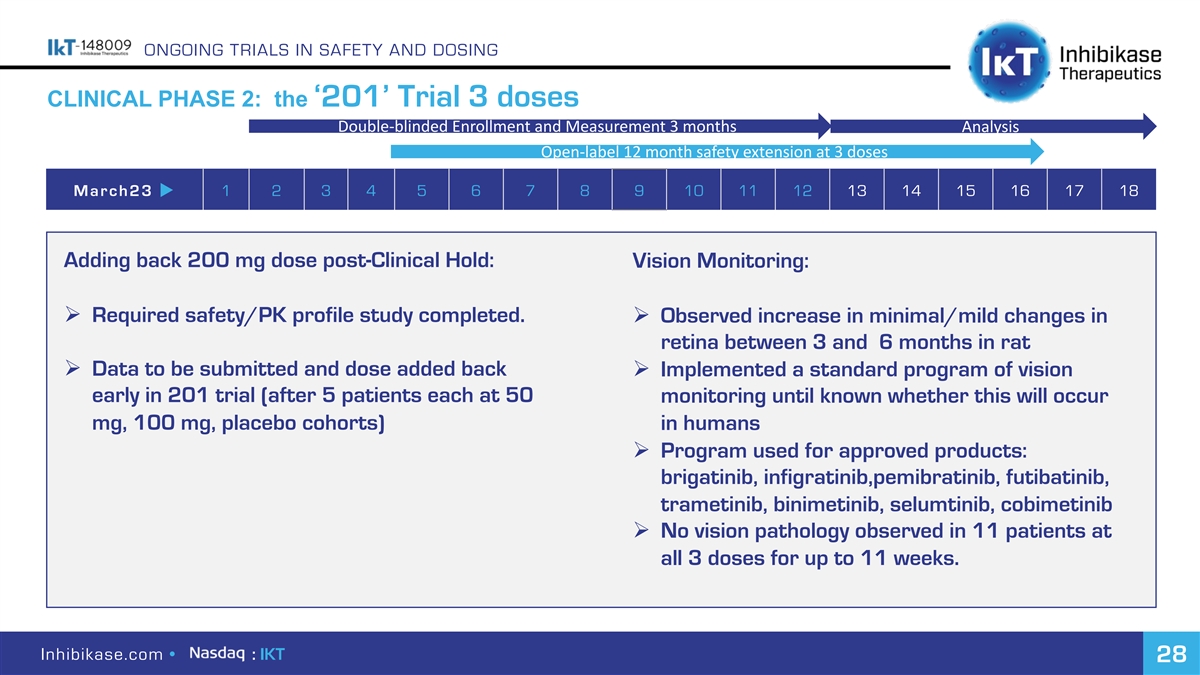
ONGOING TRIALS IN SAFETY AND DOSING CLINICAL PHASE 2: the ‘201’ Trial 3 doses Double-blinded Enrollment and Measurement 3 months Analysis Open-label 12 month safety extension at 3 doses March23 u 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Adding back 200 mg dose post-Clinical Hold: Vision Monitoring: Ø Required safety/PK profile study completed. Ø Observed increase in minimal/mild changes in retina between 3 and 6 months in rat Ø Data to be submitted and dose added back Ø Implemented a standard program of vision early in 201 trial (after 5 patients each at 50 monitoring until known whether this will occur mg, 100 mg, placebo cohorts) in humans Ø Program used for approved products: brigatinib, infigratinib,pemibratinib, futibatinib, trametinib, binimetinib, selumtinib, cobimetinib Ø No vision pathology observed in 11 patients at all 3 doses for up to 11 weeks. Inhibikase.com • : IKT 28
Clinical Development of IkT-148009 for Treatment of Multiple System Atrophy (MSA) In Inh hiib biik ka as se e.c .co om m • • :: IK IKT T 29 29
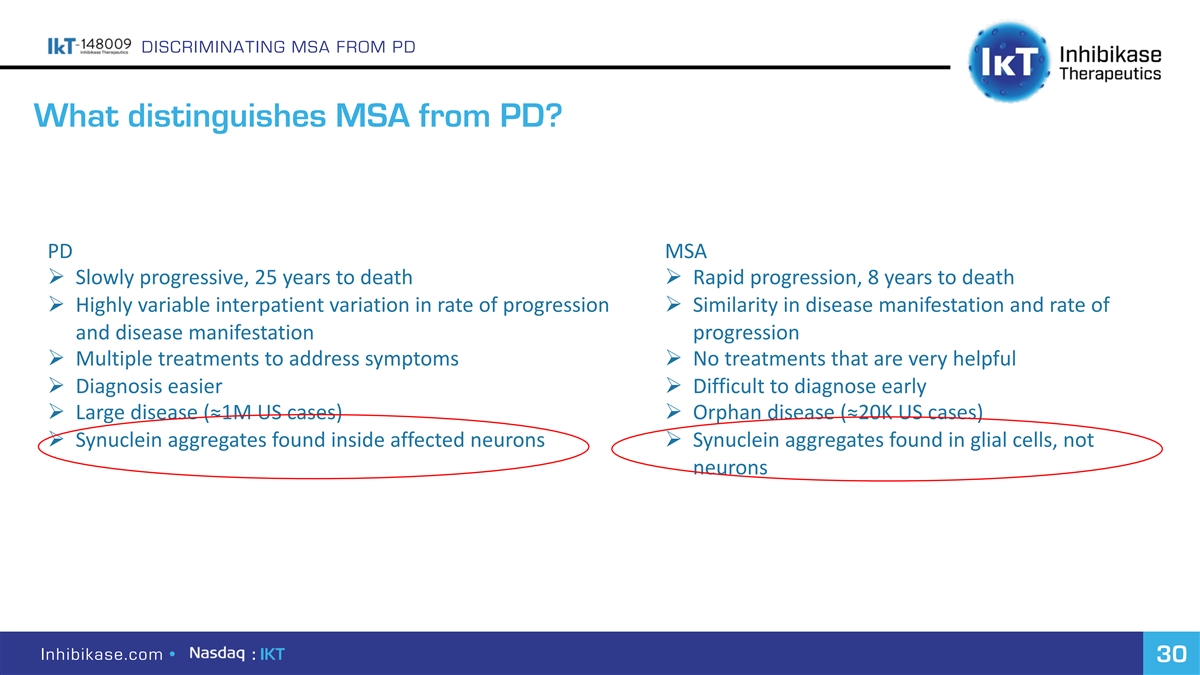
DISCRIMINATING MSA FROM PD What distinguishes MSA from PD? PD MSA Ø Slowly progressive, 25 years to death Ø Rapid progression, 8 years to death Ø Highly variable interpatient variation in rate of progression Ø Similarity in disease manifestation and rate of and disease manifestation progression Ø Multiple treatments to address symptoms Ø No treatments that are very helpful Ø Diagnosis easier Ø Difficult to diagnose early Ø Large disease (≈1M US cases) Ø Orphan disease (≈20K US cases) Ø Synuclein aggregates found inside affected neurons Ø Synuclein aggregates found in glial cells, not neurons Inhibikase.com • : IKT 30
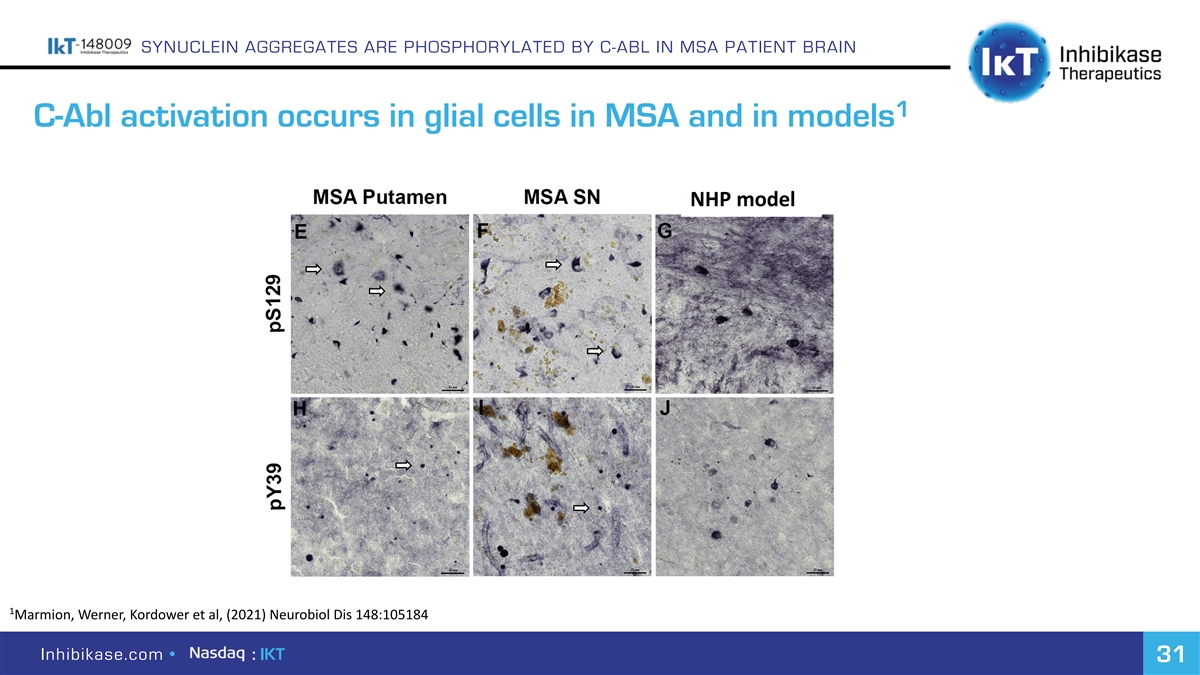
SYNUCLEIN AGGREGATES ARE PHOSPHORYLATED BY C-ABL IN MSA PATIENT BRAIN 1 C-Abl activation occurs in glial cells in MSA and in models NHP model 1 Marmion, Werner, Kordower et al, (2021) Neurobiol Dis 148:105184 Inhibikase.com • : IKT 31
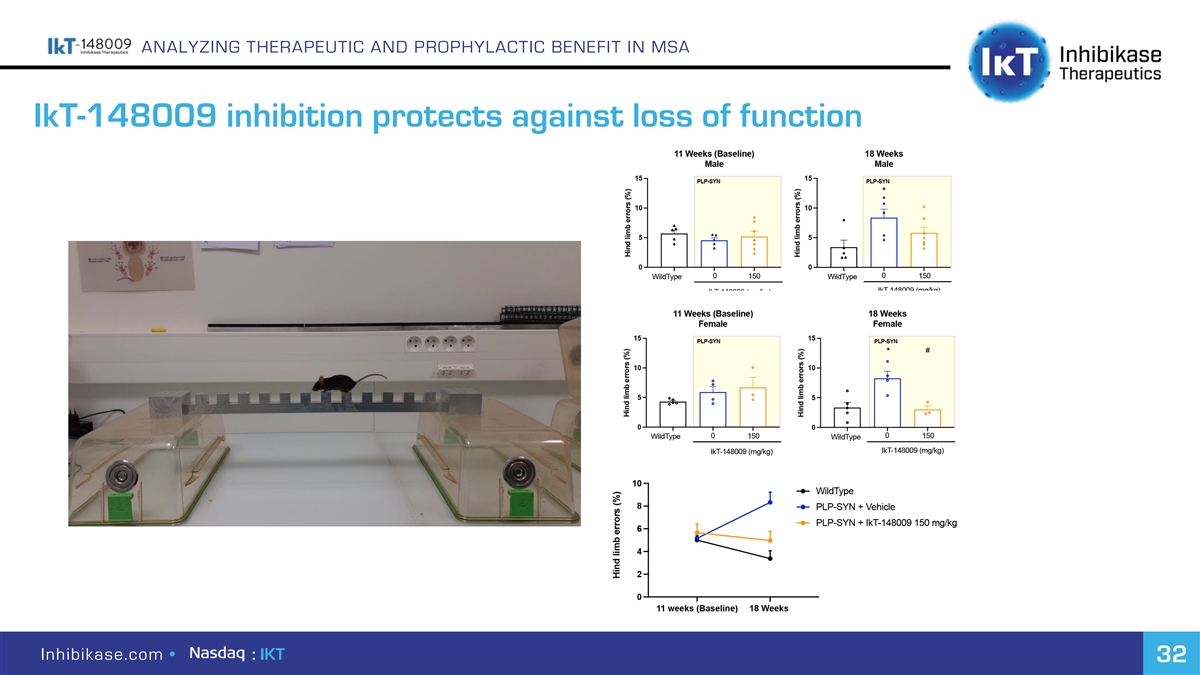
ANALYZING THERAPEUTIC AND PROPHYLACTIC BENEFIT IN MSA IkT-148009 inhibition protects against loss of function Inhibikase.com • : IKT 32
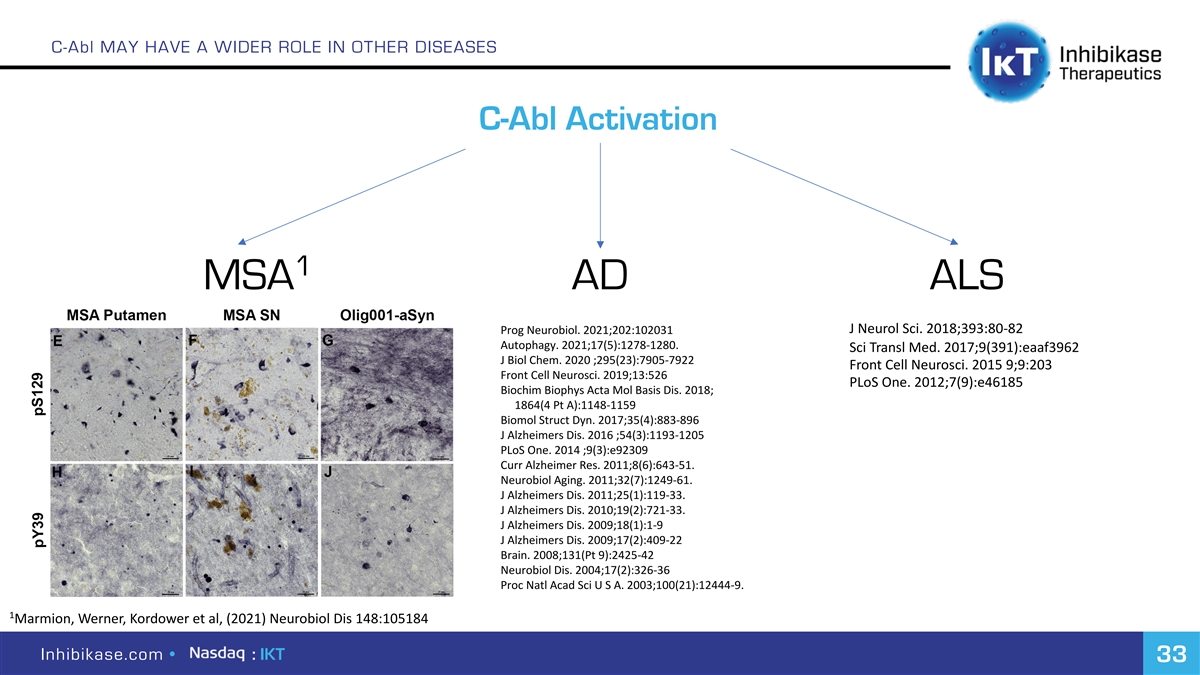
C-Abl MAY HAVE A WIDER ROLE IN OTHER DISEASES C-Abl Activation 1 MSA AD ALS Prog Neurobiol. 2021;202:102031 J Neurol Sci. 2018;393:80-82 Autophagy. 2021;17(5):1278-1280. Sci Transl Med. 2017;9(391):eaaf3962 J Biol Chem. 2020 ;295(23):7905-7922 Front Cell Neurosci. 2015 9;9:203 Front Cell Neurosci. 2019;13:526 PLoS One. 2012;7(9):e46185 Biochim Biophys Acta Mol Basis Dis. 2018; 1864(4 Pt A):1148-1159 Biomol Struct Dyn. 2017;35(4):883-896 J Alzheimers Dis. 2016 ;54(3):1193-1205 PLoS One. 2014 ;9(3):e92309 Curr Alzheimer Res. 2011;8(6):643-51. Neurobiol Aging. 2011;32(7):1249-61. J Alzheimers Dis. 2011;25(1):119-33. J Alzheimers Dis. 2010;19(2):721-33. J Alzheimers Dis. 2009;18(1):1-9 J Alzheimers Dis. 2009;17(2):409-22 Brain. 2008;131(Pt 9):2425-42 Neurobiol Dis. 2004;17(2):326-36 Proc Natl Acad Sci U S A. 2003;100(21):12444-9. 1 Marmion, Werner, Kordower et al, (2021) Neurobiol Dis 148:105184 Inhibikase.com • : IKT 33
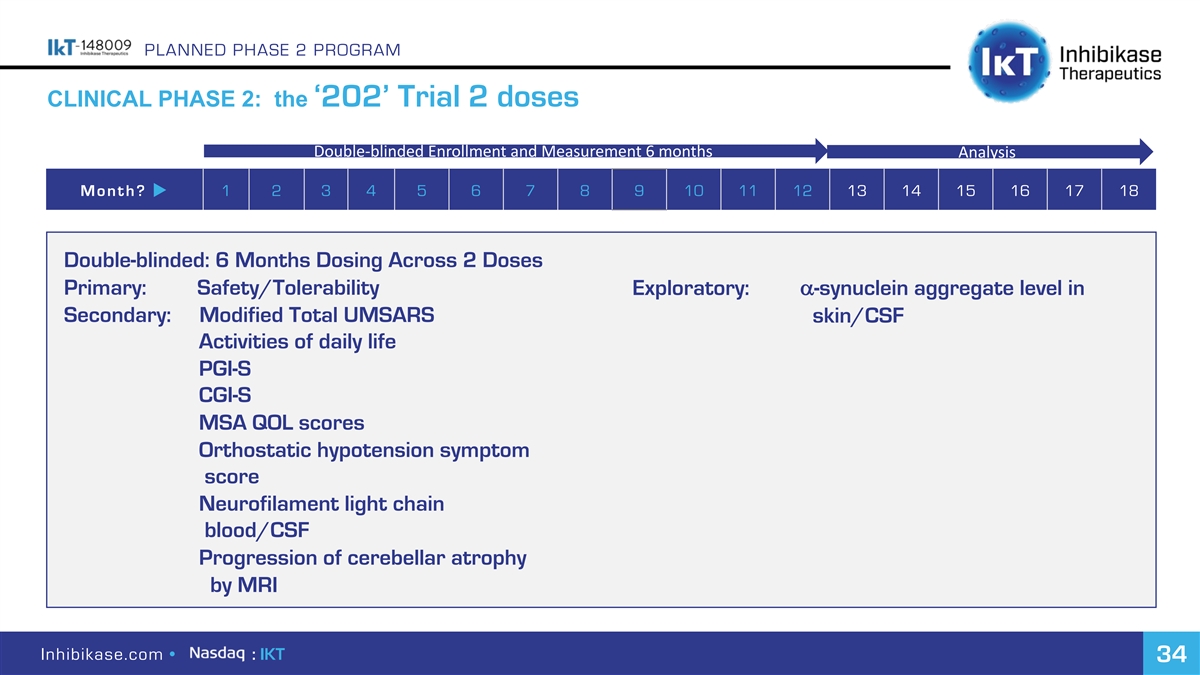
PLANNED PHASE 2 PROGRAM CLINICAL PHASE 2: the ‘202’ Trial 2 doses Double-blinded Enrollment and Measurement 6 months Analysis Month? u 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Double-blinded: 6 Months Dosing Across 2 Doses Primary: Safety/Tolerability Exploratory: a-synuclein aggregate level in Secondary: Modified Total UMSARS skin/CSF Activities of daily life PGI-S CGI-S MSA QOL scores Orthostatic hypotension symptom score Neurofilament light chain blood/CSF Progression of cerebellar atrophy by MRI Inhibikase.com • : IKT 34
2023 CATALYSTS 2023 Guidance in Neurodegeneration PD MSA Ø Fully enroll the 201 trial Ø Complete prophylactic and therapeutic animal Ø Initiate long-term extension study model studies Ø Prepare for Phase 3 program Ø Initiate MSA Phase 2 in US and EU with positive Ø Declare a commercial formulation: 2 tablet forms to be model outcome and appropriate capital tested Ø Analyze food effect on IkT-148009 Inhibikase.com • : IKT 35

Selected Stock and Financial Data In Inh hiib biik ka as se e.c .co om m • • :: IK IKT T 36 36
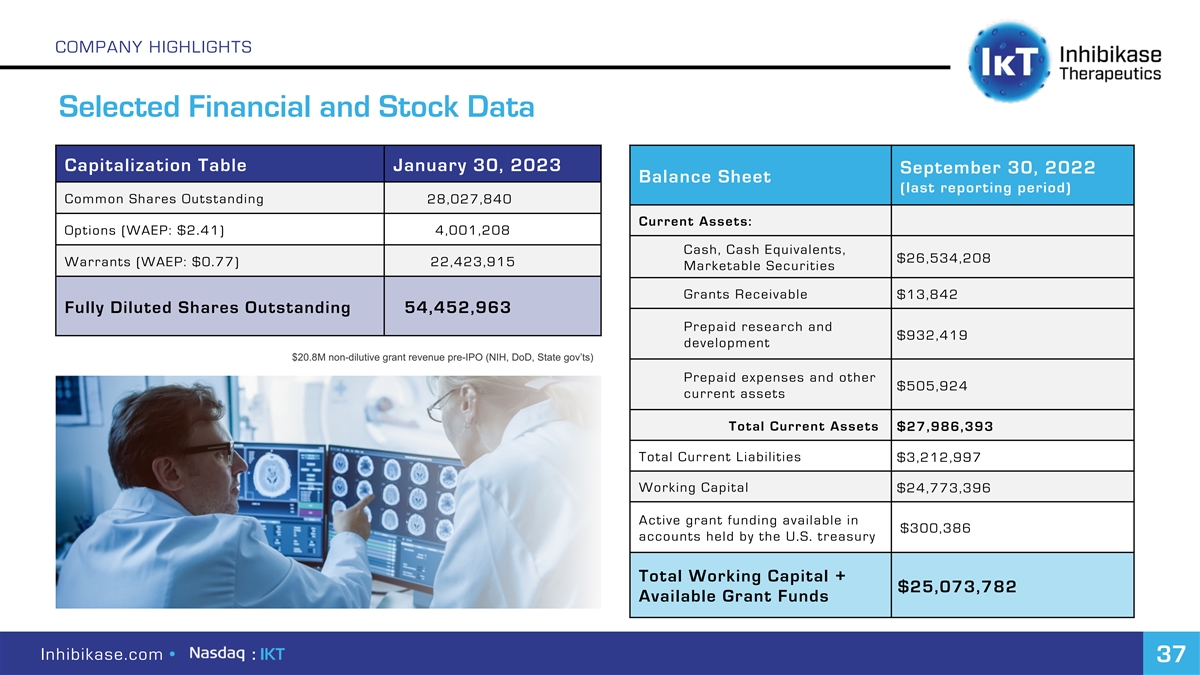
COMPANY HIGHLIGHTS Selected Financial and Stock Data Capitalization Table January 30, 2023 September 30, 2022 Balance Sheet (last reporting period) Common Shares Outstanding 28,027,840 Current Assets: Options (WAEP: $2.41) 4,001,208 Cash, Cash Equivalents, $26,534,208 Warrants (WAEP: $0.77) 22,423,915 Marketable Securities Grants Receivable $13,842 Fully Diluted Shares Outstanding 54,452,963 Prepaid research and $932,419 development $20.8M non-dilutive grant revenue pre-IPO (NIH, DoD, State gov’ts) Prepaid expenses and other $505,924 current assets Total Current Assets $27,986,393 Total Current Liabilities $3,212,997 Working Capital $24,773,396 Active grant funding available in $300,386 accounts held by the U.S. treasury Total Working Capital + $25,073,782 Available Grant Funds Inhibikase.com • : IKT 37

CONTACT E: info@inhibikase.com PH: 678-392-3419 GEORGIA OFFICE 3350 Riverwood Parkway Suite 1900 Atlanta, GA 30339 MASSACHUSETTS OFFICE 1 Cranberry Hill Suite 200 Lexington, MA 02421 Inhibikase.com : IKT