

Exhibit 99.1 IKT Parkinson’s Disease Modification Through Abl-kinase Inhibition AD-PD 2022, March 18 2022

Disclosures Dr. Milton Werner is the Founder, Chief Executive and largest shareholder of Inhibikase Therapeutics, Inc. 2 2

Disclaimer This presentation shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. This presentation contains information that may constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended. Inhibikase Therapeutics, Inc. (the “Company” or “we”) intends for the forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in those sections. Generally, we have identified such forward-looking statements by using the words “believe,” “expect,” “intend,” “estimate,” “anticipate,” “project,” “target,” “forecast,” “aim,” should, “will,” may”, “continue” and similar expressions. Such statements are subject to a number of assumptions, risks and uncertainties which may cause actual results, performance or achievements to be materially different from those anticipated in these forward- looking statements. You should read statements that contain these words carefully because they discuss future expectations and plans which contain projections of future clinical studies, regulatory approvals, product candidate development, results of operations or financial condition or state other forward-looking information. However, the absence of these words or similar expressions does not mean that a statement is not forward-looking. Forward-looking statements are not historical facts, but instead represent only the Company’s beliefs regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company’s control. It is possible that the Company’s actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward- looking statements. Management believes that these forward-looking statements are reasonable as of the time made. However, caution should be taken not to place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from the Company's historical experience and our present expectations or projections. Important factors that could cause actual results to differ materially from those in the forward-looking statements are set forth in the Company’s filings with the Securities and Exchange Commission, including its registration statement on Form S-1, as amended (File No. 333-240036), including under the caption Risk Factors. We do not intend our use or display of other entities’ names, trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity. 33

TREATING INSIDE AND OUTSIDE OF THE BRAIN Simultaneous Evaluation of Brain and GI Function Identifies a Potential Disease-Modifying Treatment BY 2025, PARKINSON’S § PD is more than just a disease of the brain, DISEASE DRUG SALES ARE EXPECTED TO § GI manifestation in many patients occurs at an early stage, indicating that evaluation of GI and brain function could be essential to identifying truly disease-modifying treatments Double Pharma Insights, 2019 § Utilization of slowly progressive, a-synuclein dependent animal models that reproduce the rate of disease progression relative to lifespan of the human disease have been key to properly SALES ESTIMATES BY identifying disease-modifying therapeutics. 2025 ARE EXPECTED TO CREST § Overview Ø Review of the role of the Abelson Tyrosine Kinase, or c-Abl, in disease $6.0 Billion Pharma Insights, 2019 Ø Demonstration of the treatment potential of c-Abl inhibitors Ø Identification of IkT-148009 as a potential disease modifying strategy THE COUNTRY WITH THE Ø Clinical safety, tolerability and measures of efficacy HIGHEST DIAGNOSED PREVALENCE IS Ø Development Timeline The U.S. DelveInsight, 2019 44
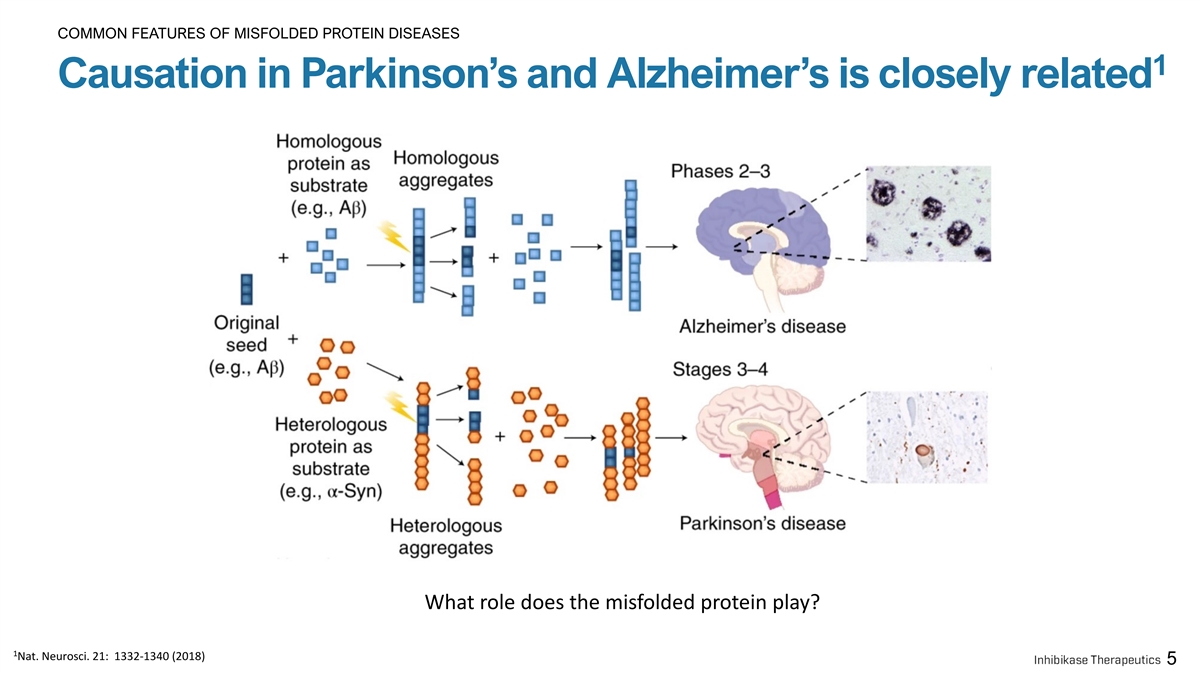
COMMON FEATURES OF MISFOLDED PROTEIN DISEASES 1 Causation in Parkinson’s and Alzheimer’s is closely related What role does the misfolded protein play? 1 Nat. Neurosci. 21: 1332-1340 (2018) 5
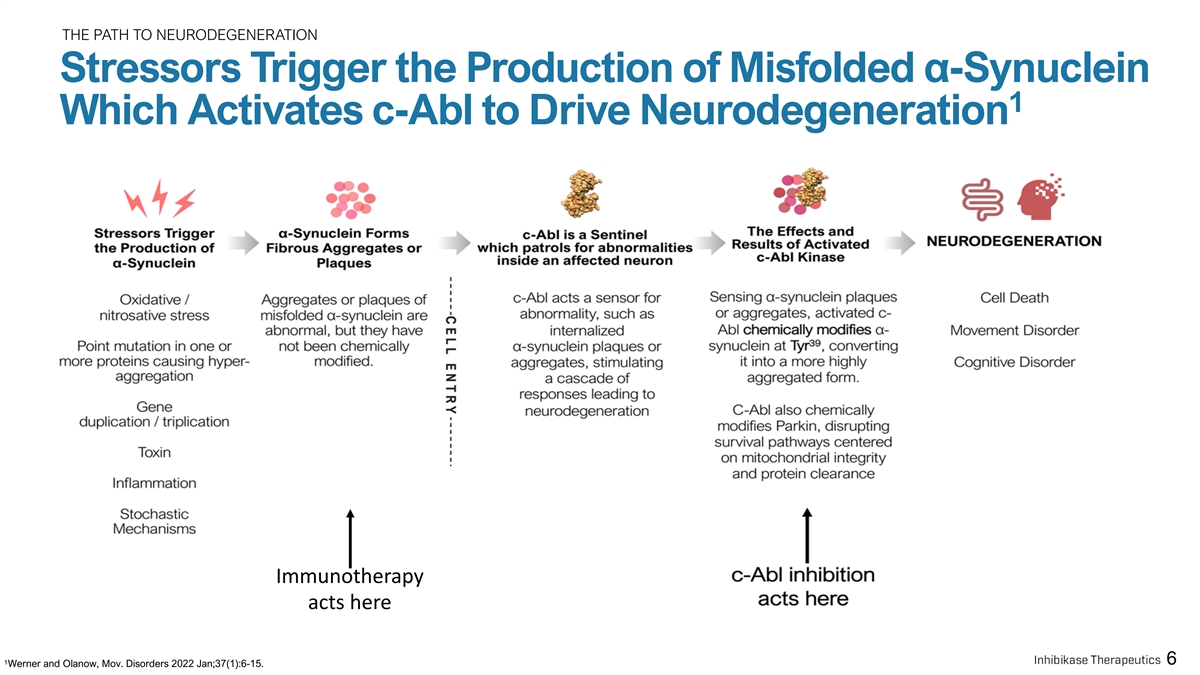
THE PATH TO NEURODEGENERATION Stressors Trigger the Production of Misfolded α-Synuclein 1 Which Activates c-Abl to Drive Neurodegeneration Immunotherapy acts here 1 6 Werner and Olanow, Mov. Disorders 2022 Jan;37(1):6-15.
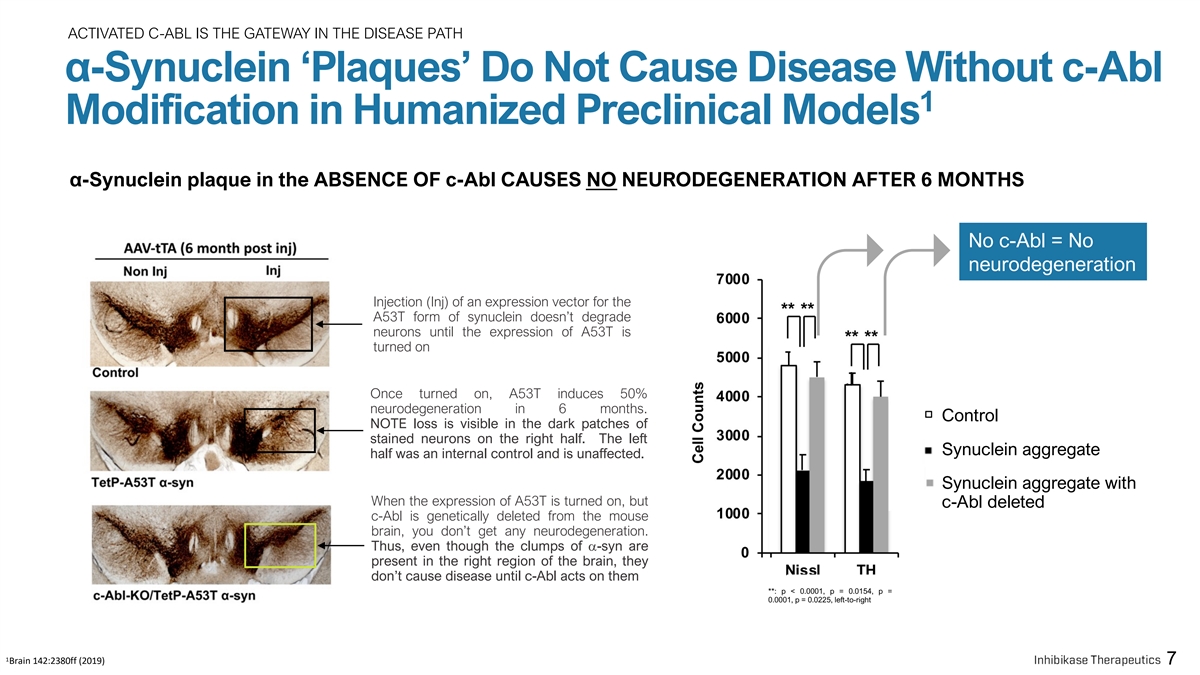
ACTIVATED C-ABL IS THE GATEWAY IN THE DISEASE PATH α-Synuclein ‘Plaques’ Do Not Cause Disease Without c-Abl 1 Modification in Humanized Preclinical Models α-Synuclein plaque in the ABSENCE OF c-Abl CAUSES NO NEURODEGENERATION AFTER 6 MONTHS No c-Abl = No neurodegeneration Injection (Inj) of an expression vector for the A53T form of synuclein doesn’t degrade neurons until the expression of A53T is turned on Once turned on, A53T induces 50% neurodegeneration in 6 months. Control NOTE loss is visible in the dark patches of stained neurons on the right half. The left Synuclein aggregate half was an internal control and is unaffected. Synuclein aggregate with When the expression of A53T is turned on, but c-Abl deleted c-Abl is genetically deleted from the mouse brain, you don’t get any neurodegeneration. Thus, even though the clumps of a-syn are present in the right region of the brain, they don’t cause disease until c-Abl acts on them **: p < 0.0001, p = 0.0154, p = 0.0001, p = 0.0225, left-to-right 1 Brain 142:2380ff (2019) 7
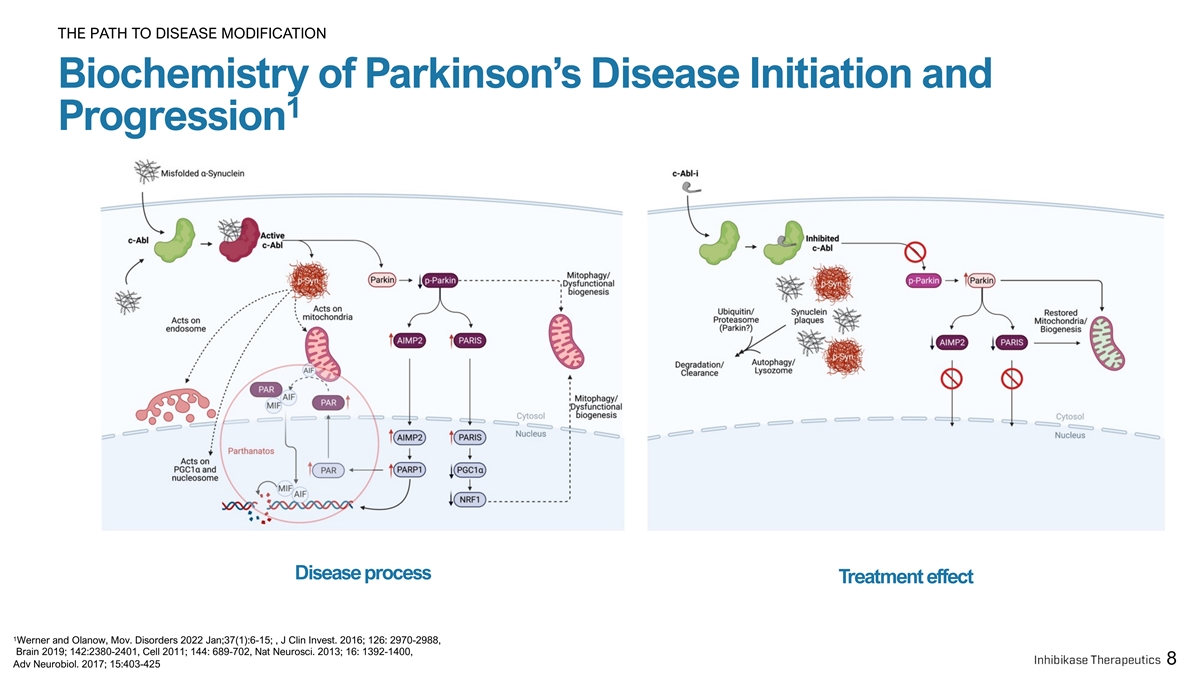
THE PATH TO DISEASE MODIFICATION Biochemistry of Parkinson’s Disease Initiation and 1 Progression Disease process Treatment effect 1 Werner and Olanow, Mov. Disorders 2022 Jan;37(1):6-15; , J Clin Invest. 2016; 126: 2970-2988, Brain 2019; 142:2380-2401, Cell 2011; 144: 689-702, Nat Neurosci. 2013; 16: 1392-1400, 8 Adv Neurobiol. 2017; 15:403-425
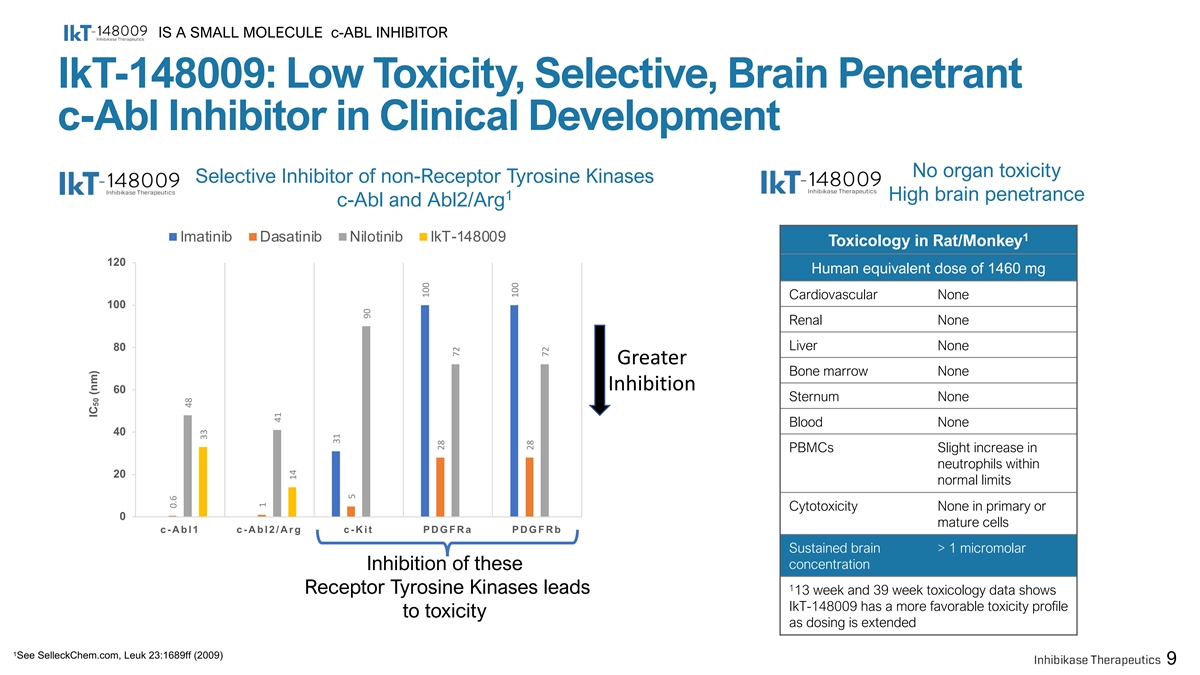
IS A SMALL MOLECULE c-ABL INHIBITOR IkT-148009: Low Toxicity, Selective, Brain Penetrant c-Abl Inhibitor in Clinical Development No organ toxicity Selective Inhibitor of non-Receptor Tyrosine Kinases 1 High brain penetrance c-Abl and Abl2/Arg Imatinib Dasatinib Nilotinib IkT-148009 1 Toxicology in Rat/Monkey 120 Human equivalent dose of 1460 mg Cardiovascular None 100 Renal None Liver None 80 Greater Bone marrow None Inhibition 60 Sternum None Blood None 40 PBMCs Slight increase in neutrophils within 20 normal limits Cytotoxicity None in primary or 0 mature cells c-Abl1 c-Abl2/Arg c-Kit PDGFRa PDGFRb Sustained brain > 1 micromolar concentration Inhibition of these 1 Receptor Tyrosine Kinases leads 13 week and 39 week toxicology data shows IkT-148009 has a more favorable toxicity profile to toxicity as dosing is extended 1 See SelleckChem.com, Leuk 23:1689ff (2009) 9 IC (nm) 50 0.6 48 33 1 41 14 31 5 90 100 28 72 100 28 72
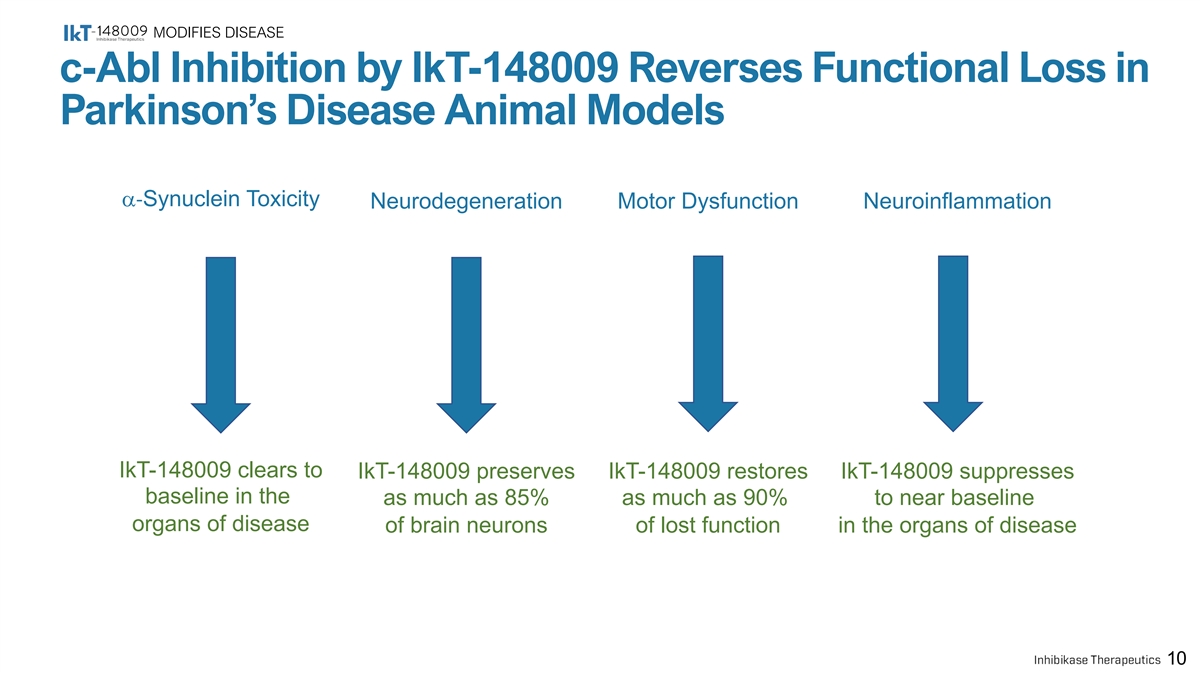
MODIFIES DISEASE c-Abl Inhibition by IkT-148009 Reverses Functional Loss in Parkinson’s Disease Animal Models a-Synuclein Toxicity Neurodegeneration Motor Dysfunction Neuroinflammation IkT-148009 clears to IkT-148009 preserves IkT-148009 restores IkT-148009 suppresses baseline in the as much as 85% as much as 90% to near baseline organs of disease of brain neurons of lost function in the organs of disease 10
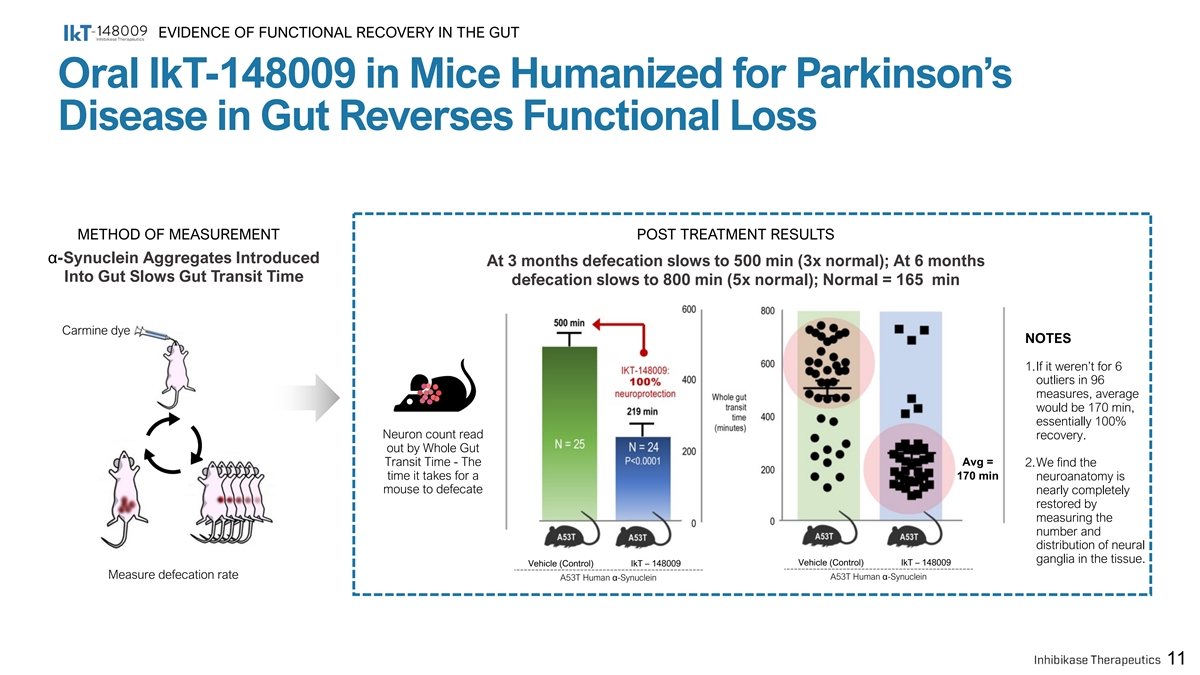
EVIDENCE OF FUNCTIONAL RECOVERY IN THE GUT Oral IkT-148009 in Mice Humanized for Parkinson’s Disease in Gut Reverses Functional Loss METHOD OF MEASUREMENT POST TREATMENT RESULTS α-Synuclein Aggregates Introduced At 3 months defecation slows to 500 min (3x normal); At 6 months Into Gut Slows Gut Transit Time defecation slows to 800 min (5x normal); Normal = 165 min Carmine dye NOTES 1.If it weren’t for 6 outliers in 96 measures, average would be 170 min, essentially 100% Neuron count read recovery. out by Whole Gut Transit Time - The Avg = 2.We find the time it takes for a 170 min neuroanatomy is mouse to defecate nearly completely restored by measuring the number and distribution of neural ganglia in the tissue. Vehicle (Control) IkT – 148009 Vehicle (Control) IkT – 148009 Measure defecation rate A53T Human α-Synuclein A53T Human α-Synuclein 11
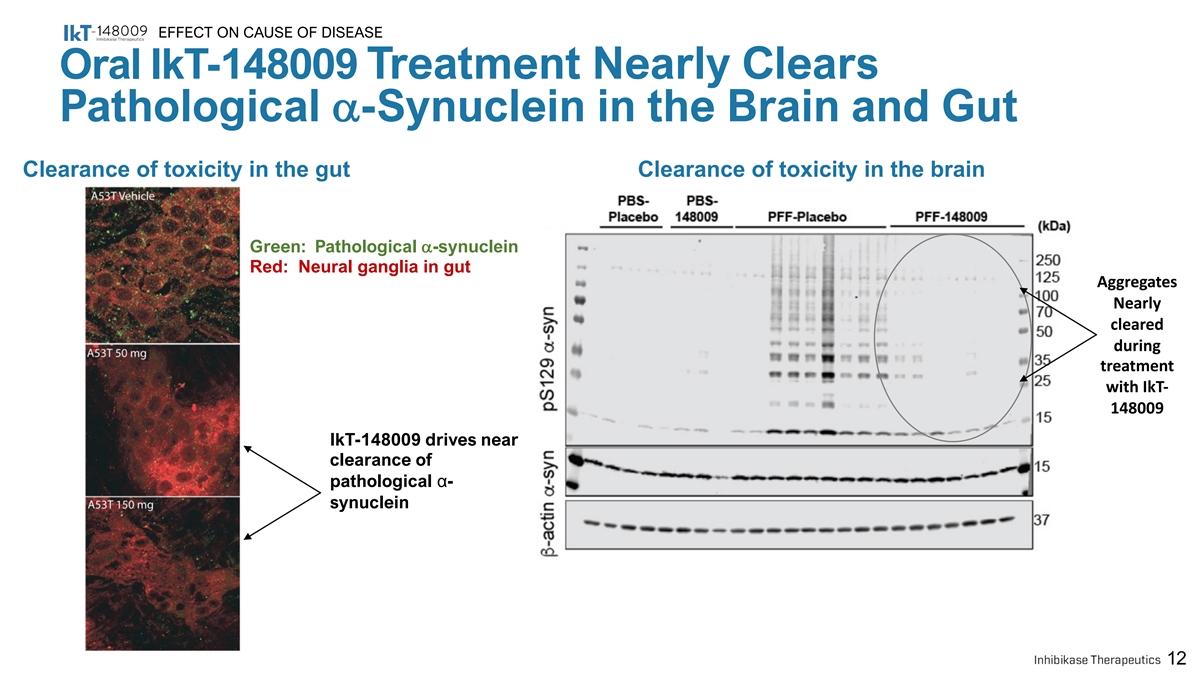
EFFECT ON CAUSE OF DISEASE Oral IkT-148009 Treatment Nearly Clears Pathological a-Synuclein in the Brain and Gut Clearance of toxicity in the gut Clearance of toxicity in the brain Green: Pathological a-synuclein Red: Neural ganglia in gut Aggregates Nearly cleared during treatment with IkT- 148009 IkT-148009 drives near clearance of pathological α- synuclein 12

SUMMARY Key points of Parkinson’s Disease Initiation and 1 Progression § Internalization of misfolded or aggregated a-synuclein and the activation of c-Abl in response is a key event in PD initiation/progression § The long-sought goal of clearing a-synuclein aggregates for a therapeutic purpose should focus on the aggregates WITHIN the affected neurons § Reduction and/or clearance of aggregates can be driven by restoring endogenous processes from within the affected neurons § Recovery of lost functional activity can be achieved 1 Werner and Olanow, Mov. Disorders 2022 Jan;37(1):6-15; , J Clin Invest. 2016; 126: 2970-2988, Brain 2019; 142:2380-2401, Cell 2011; 144: 689-702, Nat Neurosci. 2013; 16: 1392-1400, 13 Adv Neurobiol. 2017; 15:403-425

IKT Clinical Development and Status AD-PD 2022, March 18 2022
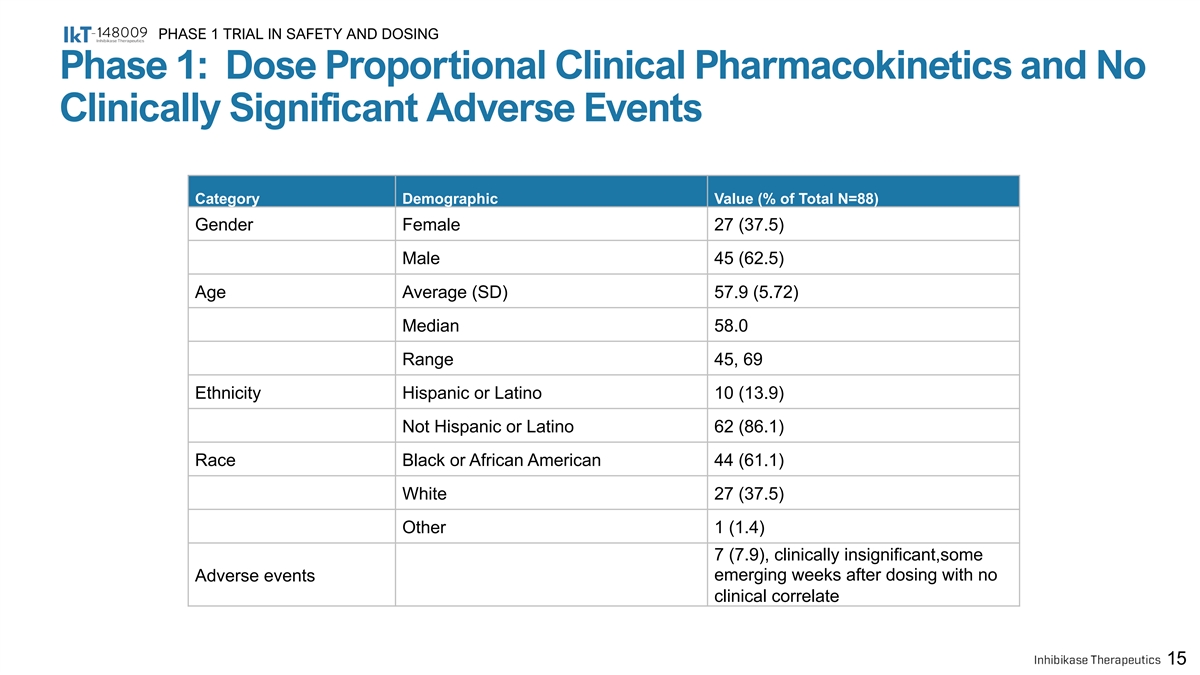
PHASE 1 TRIAL IN SAFETY AND DOSING Phase 1: Dose Proportional Clinical Pharmacokinetics and No Clinically Significant Adverse Events Category Demographic Value (% of Total N=88) Gender Female 27 (37.5) Male 45 (62.5) Age Average (SD) 57.9 (5.72) Median 58.0 Range 45, 69 Ethnicity Hispanic or Latino 10 (13.9) Not Hispanic or Latino 62 (86.1) Race Black or African American 44 (61.1) White 27 (37.5) Other 1 (1.4) 7 (7.9), clinically insignificant,some Adverse events emerging weeks after dosing with no clinical correlate 15
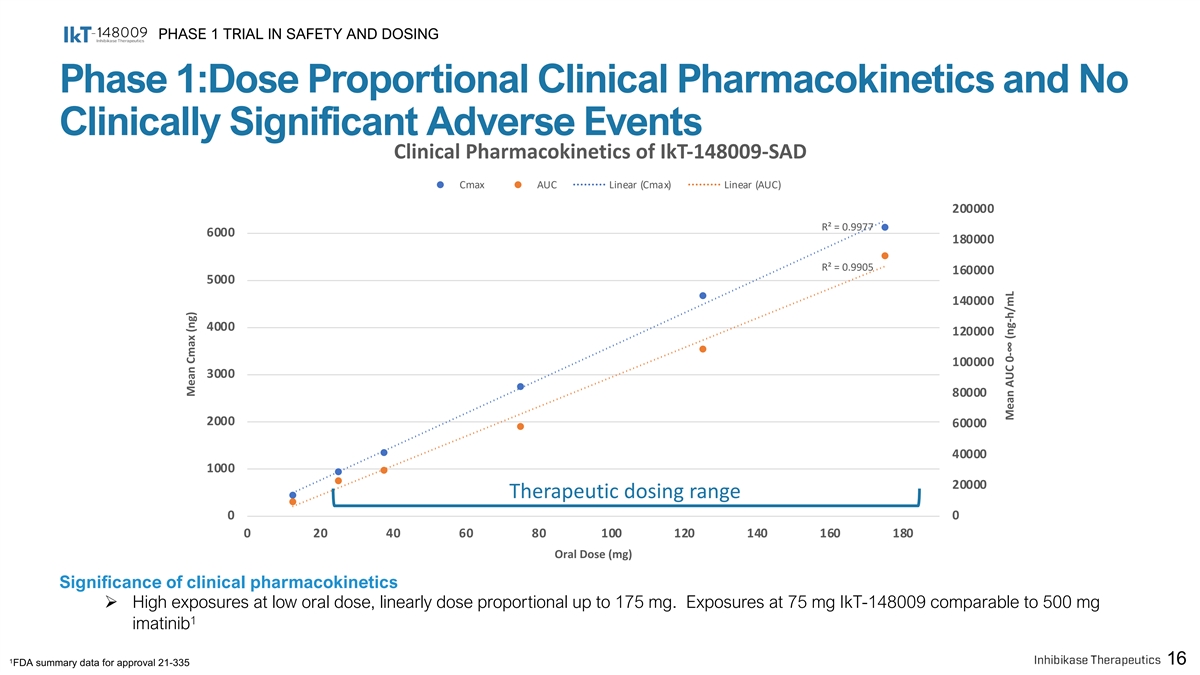
PHASE 1 TRIAL IN SAFETY AND DOSING Phase 1:Dose Proportional Clinical Pharmacokinetics and No Clinically Significant Adverse Events Clinical Pharmacokinetics of IkT-148009-SAD Cmax AUC Linear (Cmax) Linear (AUC) 200000 R² = 0.9977 6000 180000 R² = 0.9905 160000 5000 140000 4000 120000 100000 3000 80000 2000 60000 40000 1000 20000 Therapeutic dosing range 0 0 0 20 40 60 80 100 120 140 160 180 Oral Dose (mg) Significance of clinical pharmacokinetics Ø High exposures at low oral dose, linearly dose proportional up to 175 mg. Exposures at 75 mg IkT-148009 comparable to 500 mg 1 imatinib 1 16 FDA summary data for approval 21-335 Mean Cmax (ng) Mean AUC 0-∞ (ng-h/mL
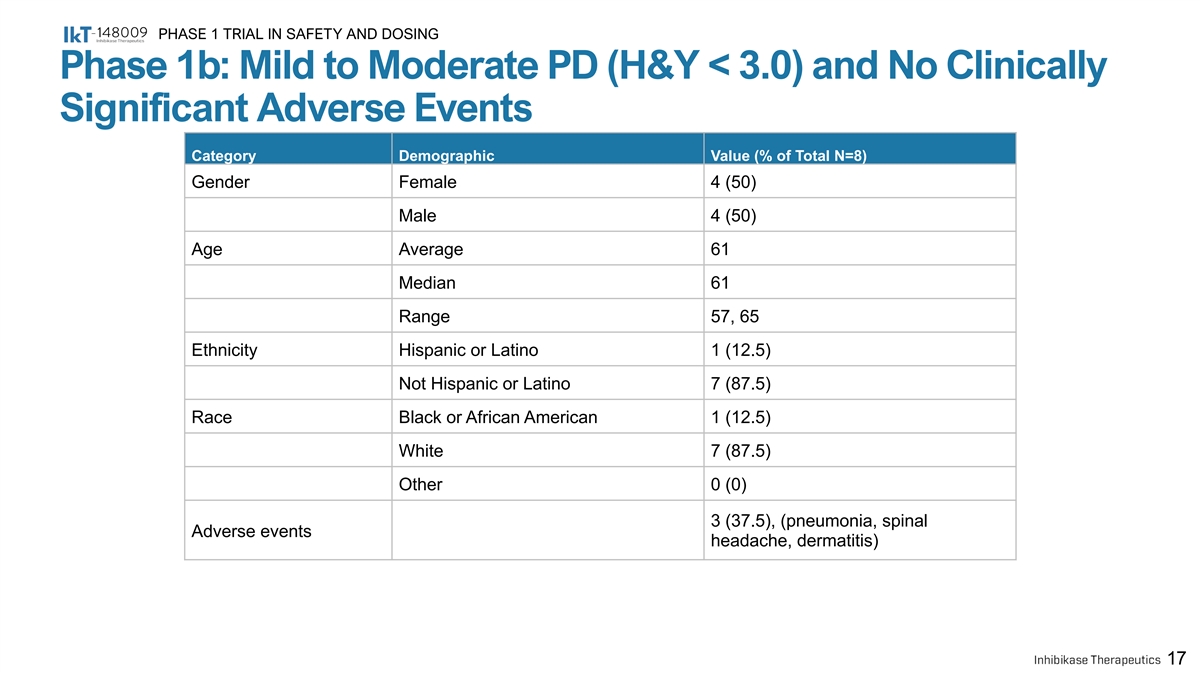
PHASE 1 TRIAL IN SAFETY AND DOSING Phase 1b: Mild to Moderate PD (H&Y < 3.0) and No Clinically Significant Adverse Events Category Demographic Value (% of Total N=8) Gender Female 4 (50) Male 4 (50) Age Average 61 Median 61 Range 57, 65 Ethnicity Hispanic or Latino 1 (12.5) Not Hispanic or Latino 7 (87.5) Race Black or African American 1 (12.5) White 7 (87.5) Other 0 (0) 3 (37.5), (pneumonia, spinal Adverse events headache, dermatitis) 17
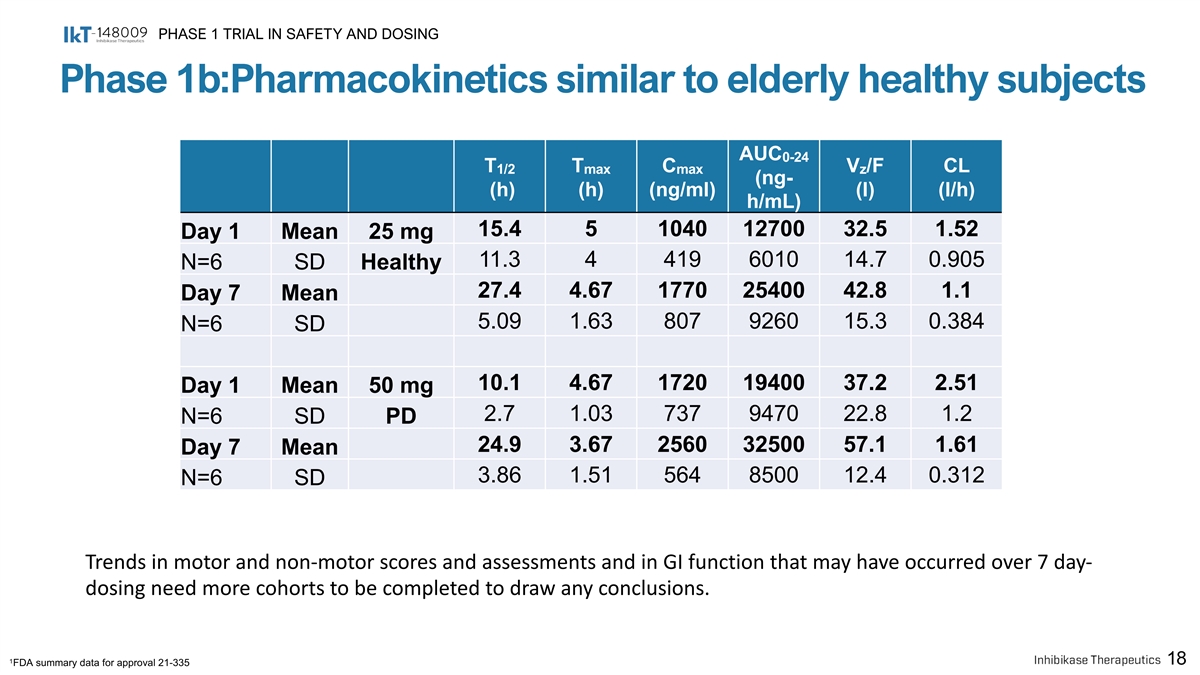
PHASE 1 TRIAL IN SAFETY AND DOSING Phase 1b:Pharmacokinetics similar to elderly healthy subjects AUC 0-24 T T C V /F CL 1/2 max max z (ng- (h) (h) (ng/ml) (l) (l/h) h/mL) 15.4 5 1040 12700 32.5 1.52 Day 1 Mean 25 mg 11.3 4 419 6010 14.7 0.905 N=6 SD Healthy 27.4 4.67 1770 25400 42.8 1.1 Day 7 Mean 5.09 1.63 807 9260 15.3 0.384 N=6 SD 10.1 4.67 1720 19400 37.2 2.51 Day 1 Mean 50 mg 2.7 1.03 737 9470 22.8 1.2 N=6 SD PD 24.9 3.67 2560 32500 57.1 1.61 Day 7 Mean 3.86 1.51 564 8500 12.4 0.312 N=6 SD Trends in motor and non-motor scores and assessments and in GI function that may have occurred over 7 day- dosing need more cohorts to be completed to draw any conclusions. 1 18 FDA summary data for approval 21-335
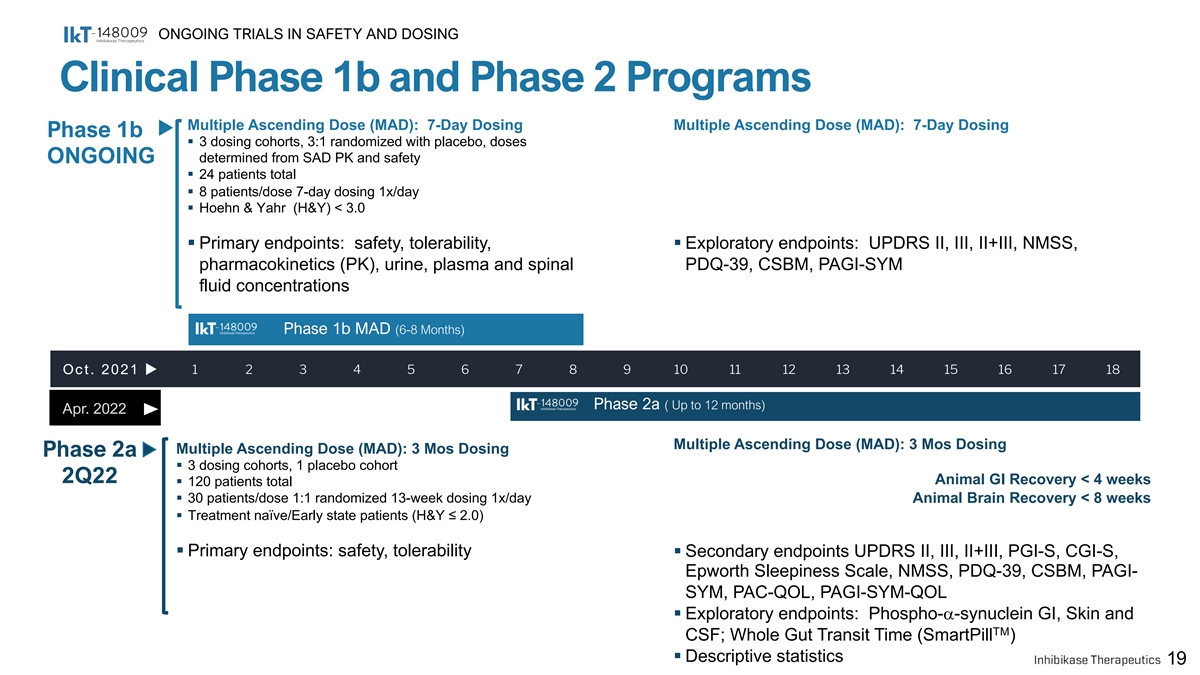
ONGOING TRIALS IN SAFETY AND DOSING Clinical Phase 1b and Phase 2 Programs Multiple Ascending Dose (MAD): 7-Day Dosing Multiple Ascending Dose (MAD): 7-Day Dosing Phase 1b § 3 dosing cohorts, 3:1 randomized with placebo, doses determined from SAD PK and safety ONGOING § 24 patients total § 8 patients/dose 7-day dosing 1x/day § Hoehn & Yahr (H&Y) < 3.0 § Primary endpoints: safety, tolerability, § Exploratory endpoints: UPDRS II, III, II+III, NMSS, pharmacokinetics (PK), urine, plasma and spinal PDQ-39, CSBM, PAGI-SYM fluid concentrations Phase 1b MAD (6-8 Months) Oct. 2021 u 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Phase 2a ( Up to 12 months) Apr. 2022 Multiple Ascending Dose (MAD): 3 Mos Dosing Multiple Ascending Dose (MAD): 3 Mos Dosing Phase 2a § 3 dosing cohorts, 1 placebo cohort 2Q22 Animal GI Recovery < 4 weeks § 120 patients total § 30 patients/dose 1:1 randomized 13-week dosing 1x/day Animal Brain Recovery < 8 weeks § Treatment naïve/Early state patients (H&Y ≤ 2.0) § Primary endpoints: safety, tolerability § Secondary endpoints UPDRS II, III, II+III, PGI-S, CGI-S, Epworth Sleepiness Scale, NMSS, PDQ-39, CSBM, PAGI- SYM, PAC-QOL, PAGI-SYM-QOL § Exploratory endpoints: Phospho-a-synuclein GI, Skin and TM CSF; Whole Gut Transit Time (SmartPill ) § Descriptive statistics 19

ACKNOWLEDGEMENTS Multi-institutional effort across five models Inhibikase Therapeutics, Inc. Parkinson’s Institute Johns Hopkins University Senthil Karuppagounder, PhD Milton H Werner PhD Tyler Molitor, PhD Richard Nguyen MS Roger Rush PhD Cassandra Hempel Shivani Bisen Terence Kelly, PhD Ashani Chand Yoko Yamashita Surendra Singh PhD Carolee Barlow, MD Nicholas Sloan Brianna Dang Sydney Kruger Alexander Sigmon Irena Webster, MPH Yyeun Woo Lee Warren Olanow, MD Shirley Marino Lee Leslie Watkins Andrew McGarry,MD Erica Kim Cornelia Kamp Saurav Brahmachari, PhD Syner-G Bio Manoj Kumar, PhD Voisin Consulting Hu Wang, PhD Ted Dawson, MD PhD Valina Dawson, PhD Subhash Kulkarni, PhD Qian Li, PhD Jay Pasricha, MD Funding: MJFF 13682, NINDS NS103695 20

Q&A Targeting c-Abl we believe is transformational to treatment of neurodegeneration 21