
Exhibit 99.1

Clinical Development of Therapies Intended to Reverse the Functional Loss in Parkinson’s and Related Disorders 1Q 2022 BUSINESS PRESENTATION IKT

2 This presentation shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction . This presentation contains information that may constitute “forward - looking statements” within the meaning of Section 27 A of the Securities Act, and Section 21 E of the Securities Exchange Act of 1934 , as amended . Inhibikase Therapeutics, Inc . (the “Company” or “we”) intends for the forward - looking statements to be covered by the safe harbor provisions for forward - looking statements in those sections . Generally, we have identified such forward - looking statements by using the words “believe,” “expect,” “intend,” “estimate,” “anticipate,” “project,” “target,” “forecast,” “aim,” "should," “will,” "may”, “continue” and similar expressions . Such statements are subject to a number of assumptions, risks and uncertainties which may cause actual results, performance or achievements to be materially different from those anticipated in these forward - looking statements . You should read statements that contain these words carefully because they discuss future expectations and plans which contain projections of future clinical studies, regulatory approvals, product candidate development, results of operations or financial condition or state other forward - looking information . However, the absence of these words or similar expressions does not mean that a statement is not forward - looking . Forward - looking statements are not historical facts, but instead represent only the Company’s beliefs regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company’s control . It is possible that the Company’s actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward - looking statements . Management believes that these forward - looking statements are reasonable as of the time made . However, caution should be taken not to place undue reliance on any such forward - looking statements because such statements speak only as of the date when made . The Company undertakes no obligation to publicly update or revise any forward - looking statements, whether as a result of new information, future events or otherwise, except as required by law . In addition, forward - looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from the Company's historical experience and our present expectations or projections . Important factors that could cause actual results to differ materially from those in the forward - looking statements are set forth in the Company’s filings with the Securities and Exchange Commission, including its registration statement on Form S - 1 , as amended (File No . 333 - 240036 ), including under the caption "Risk Factors . We do not intend our use or display of other entities’ names, trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity . D i s c l a i m e r

COMPANY HIGHLIGHTS Driving Functional Reversal of Parkinson’s Disease ▪ Multi - therapeutic pipeline with emphasis on neurodegeneration inside and outside of the brain ▪ Our lead inhibitor of the Abelson Tyrosine Kinase (c - Abl), IkT - 148009, halts and reverses functional loss in animal models that recreate progressive human disease ▪ Six clinical programs in 2022 across two assets in Parkinson’s disease, Multiple Systems Atrophy (orphan indication, orphan designation not sought) and Chronic Myelogenous Leukemia (orphan indication, orphan designation granted). ▪ Phase 2 programs anticipated to begin early 2Q22 in neurodegeneration, readouts anticipated within 12 months. ▪ Multiple patent families for lead compound with expiration of 2036 and beyond ▪ $20.8 million in grants and contracts from NIH, DoD, the Michael J. Fox Foundation and the Georgia Research Alliance, all peer - reviewed ▪ $63 million gross proceeds in investor capital in 2021 ▪ Highly experienced and respected management team, consultants, Board of Directors and nearly all KOLs in the field on Scientific Advisory Board SALES ESTIMATES BY 2025 ARE EXPECTED TO CREST BY 2025, PARKINSON’S DISEASE DRUG SALES ARE EXPECTED TO Double Pharma Insights, 2019 $6.0 Billion Pharma Insights , 2019 THE COUNTRY WITH THE HIGHEST DIAGNOSED PREVALENCE IS The U.S. DelveInsight, 2019 3 3
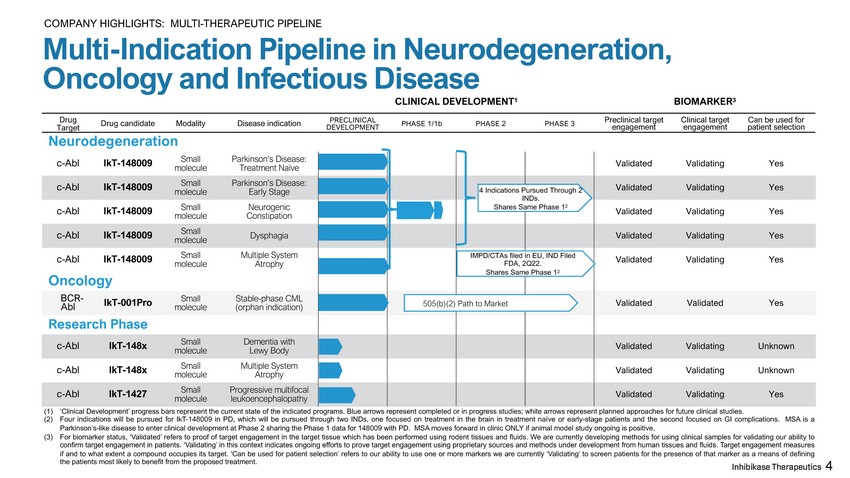
BIOMARKER 3 Multi - Indication Pipeline in Neurodegeneration, Oncology and Infectious Disease CLINICAL DEVELOPMENT 1 D r u g Ta r g e t Drug candidate Modality Disease indication PRECLINICAL D E V E L O P M E N T PHASE 1/1b PHASE 2 PHASE 3 Preclinical target engagement Clinical target engagement Can be used for patient selection Neurodegeneration Small m o l e c u l e Parkinson’s Disease: Treatment Naïve Validated Validating Y e s Small m o l e c u l e Parkinson’s Disease: Early Stage Validated Validating Y e s Small m o l e c u l e Neurogenic C o n s t i p at i o n Validated Validating Y e s Small m o l e c u l e D y s p h a g i a Validated Validating Y e s Small m o l e c u l e Multiple System Atrophy Validated Validating Y e s c - Abl IkT - 148009 c - Abl IkT - 148009 c - Abl IkT - 148009 c - Abl IkT - 148009 c - Abl IkT - 148009 Oncology BC R - Abl IkT - 001Pro Small m o l e c u l e Stable - phase CML (orphan indication) Validated Validated Y e s Small m o l e c u l e Dementia with Lewy Body Validated Validating U n k no wn Small m o l e c u l e Multiple System Atrophy Validated Validating U n k no wn Research Phase c - Abl IkT - 148x c - Abl IkT - 148x c - Abl IkT - 1427 Small m o l e c u l e Progressive multifocal leukoencephalopathy V a li d a t e d V a li d a t i n g Y e s (1) ‘Clinical Development’ progress bars represent the current state of the indicated programs . Blue arrows represent completed or in progress studies ; white arrows represent planned approaches for future clinical studies . (2) Four indications will be pursued for IkT - 148009 in PD, which will be pursued through two INDs, one focused on treatment in the brain in treatment naïve or early - stage patients and the second focused on GI complications . MSA is a Parkinson’s - like disease to enter clinical development at Phase 2 sharing the Phase 1 data for 148009 with PD . MSA moves forward in clinic ONLY if animal model study ongoing is positive . (3) For biomarker status, ‘Validated’ refers to proof of target engagement in the target tissue which has been performed using rodent tissues and fluids . We are currently developing methods for using clinical samples for validating our ability to confirm target engagement in patients . ‘Validating’ in this context indicates ongoing efforts to prove target engagement using proprietary sources and methods under development from human tissues and fluids . Target engagement measures if and to what extent a compound occupies its target . ‘Can be used for patient selection’ refers to our ability to use one or more markers we are currently ‘Validating’ to screen patients for the presence of that marker as a means of defining the patients most likely to benefit from the proposed treatment . 4 Indications Pursued Through 2 INDs. Shares Same Phase 1 2 505(b)(2) Path to Market IMPD/CTAs filed in EU, IND Filed FDA, 2Q22. Shares Same Phase 1 2 4 COMPANY HIGHLIGHTS: MULTI - THERAPEUTIC PIPELINE

Parkinson’s Disease Market & Strategy IKT
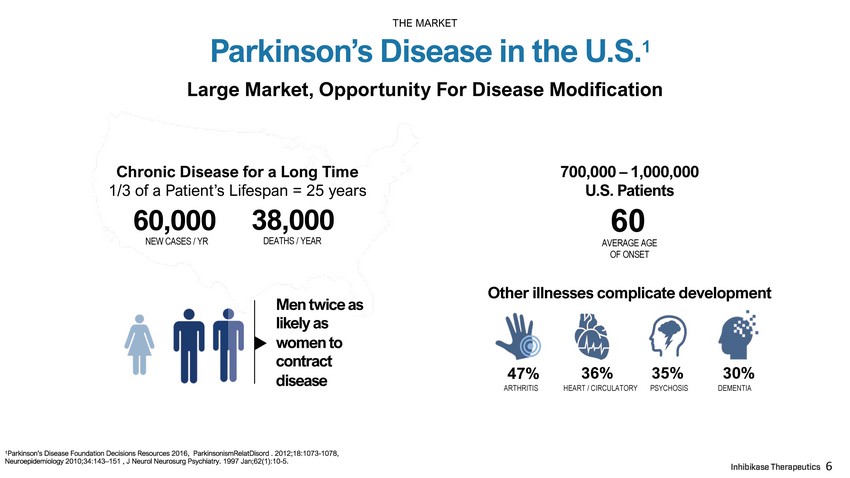
Large Market, Opportunity For Disease Modification 700,000 – 1,000,000 U.S. Patients 60 AVERAGE AGE OF ONSET 60 , 000 NEW CASES / YR Other illnesses complicate development 47% 36% 35% 30% A R T HR I T I S HEART / CIRCULATORY PSYCHOSIS DEMENTIA 38 , 000 DEATHS / YEAR Chronic Disease for a Long Time 1/3 of a Patient’s Lifespan = 25 years Men twice as likely as women to contract disease Parkinson’s Disease in the U.S. 1 THE MARKET 6 1 Parkinson’s Disease Foundation Decisions Resources 2016, ParkinsonismRelatDisord . 2012;18:1073 - 1078, Neuroepidemiology 2010;34:143 – 151 , J Neurol Neurosurg Psychiatry. 1997 Jan;62(1):10 - 5.
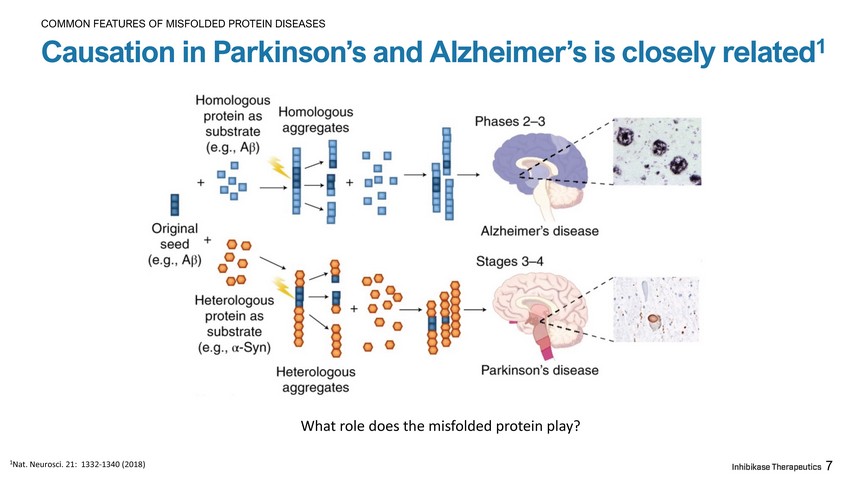
7 Causation in Parkinson’s and Alzheimer’s is closely related 1 COMMON FEATURES OF MISFOLDED PROTEIN DISEASES What role does the misfolded protein play? 1 Nat. Neurosci. 21: 1332 - 1340 (2018)

CAUSE OF NEURODEGENERATION Evaluation of the Misfolded Protein ‘Seed’ in Parkinson’s Reveals c - Abl as the Primary Culprit (video) 8
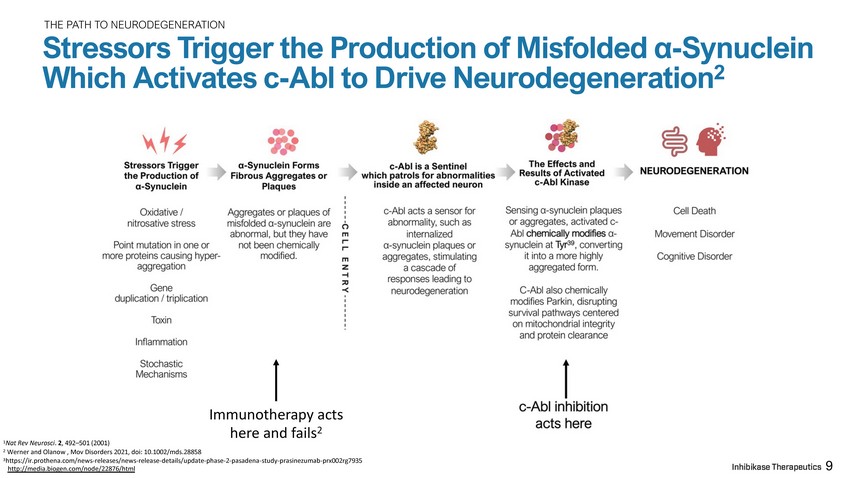
THE PATH TO NEURODEGENERATION Stressors Trigger the Production of Misfolded α - Synuclein Which Activates c - Abl to Drive Neurodegeneration 2 1 Nat Rev Neurosci . 2 , 492 – 501 (2001) 2 Werner and Olanow , Mov Disorders 2021, doi: 10.1002/mds.28858 3 https://ir.prothena.com/news - releases/news - release - details/update - phase - 2 - pasadena - study - prasinezumab - prx002rg7935 http://media.biogen.com/node/22876/htm l 9 Immunotherapy acts here and fails 2

How Inhibikase Will Modify Disease IKT
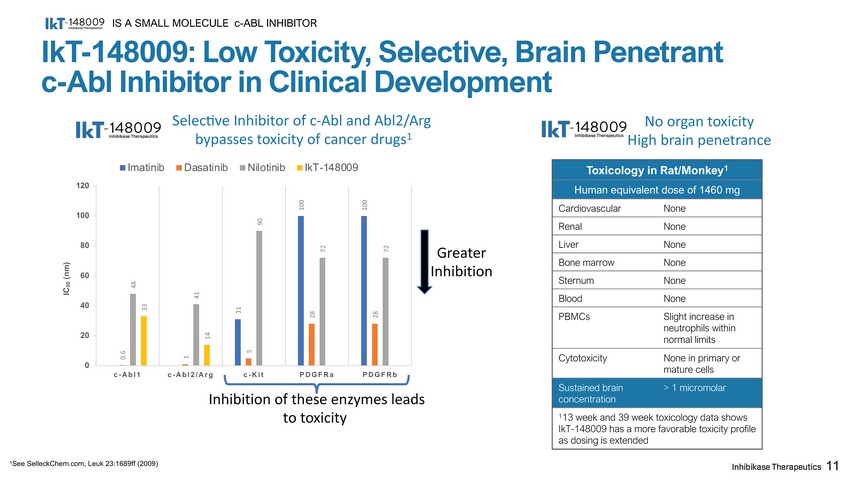
IkT - 148009: Low Toxicity, Selective, Brain Penetrant c - Abl Inhibitor in Clinical Development Toxicology in Rat/Monkey 1 Human equivalent dose of 1460 mg Cardiovascular None Renal None Liver None Bone marrow None Sternum None Blood None PBMCs Slight increase in neutrophils within normal limits Cytotoxicity None in primary or mature cells Sustained brain concentration > 1 micromolar 1 13 week and 39 week toxicology data shows IkT - 148009 has a more favorable toxicity profile as dosing is extended IS A SMALL MOLECULE c - ABL INHIBITOR 11 No organ toxicity High brain penetrance 1 See SelleckChem.com, Leuk 23:1689ff (2009) 31 100 100 0.6 1 5 28 28 48 41 90 72 72 33 14 c - Abl2/Ar g c - Kit PDGFRa PDGFRb Inhibition of these enzymes leads to toxicity 0 40 20 60 80 120 100 c - Abl1 IC 50 (nm) Imatinib Selec7ve Inhibitor of c - Abl and Abl2/Arg bypasses toxicity of cancer drugs 1 Dasatinib Nilotinib IkT - 148009 Greater I n h i b it ion
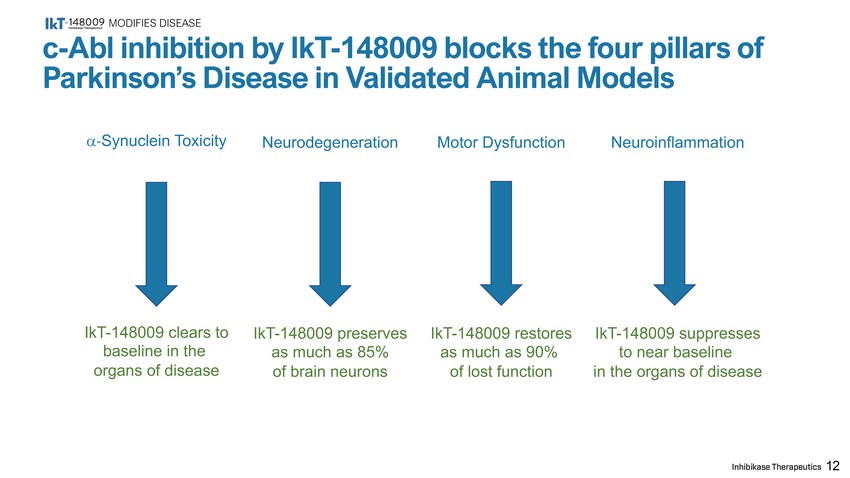
MODIFIES DISEASE 12 c - Abl inhibition by IkT - 148009 blocks the four pillars of Parkinson’s Disease in Validated Animal Models - Synuclein Toxicity IkT - 148009 clears to baseline in the organs of disease Neurodegeneration IkT - 148009 preserves as much as 85% of brain neurons Motor Dysfunction IkT - 148009 restores as much as 90% of lost function Neuroinflammation IkT - 148009 suppresses to near baseline in the organs of disease

Clinical Development IKT
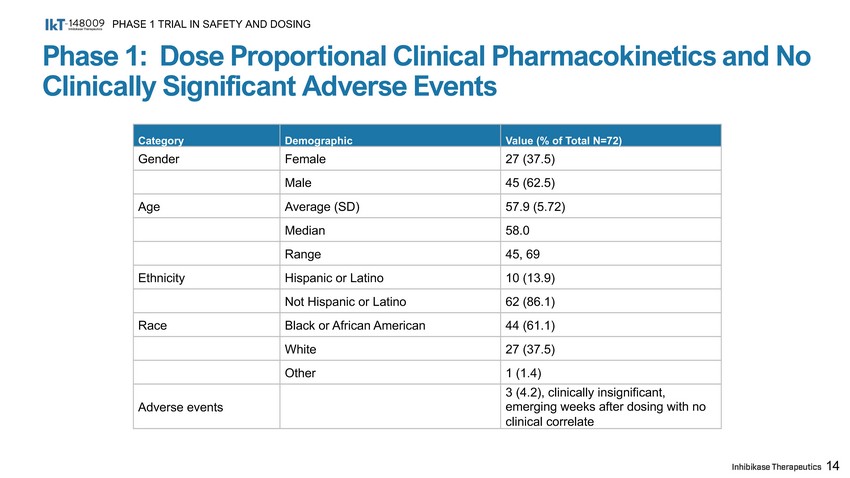
Phase 1: Dose Proportional Clinical Pharmacokinetics and No Clinically Significant Adverse Events PHASE 1 TRIAL IN SAFETY AND DOSING 14 Category Demographic Value (% of Total N=72) Gender Female 27 (37.5) Male 45 (62.5) Age Average (SD) 57.9 (5.72) Median 58.0 Range 45, 69 Ethnicity Hispanic or Latino 10 (13.9) Not Hispanic or Latino 62 (86.1) Race Black or African American 44 (61.1) White 27 (37.5) Other 1 (1.4) Adverse events 3 (4.2), clinically insignificant, emerging weeks after dosing with no clinical correlate
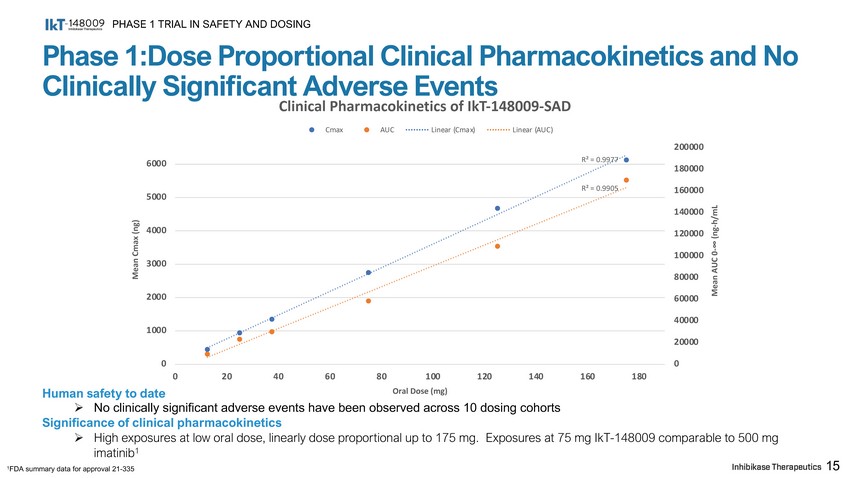
Phase 1:Dose Proportional Clinical Pharmacokinetics and No Clinically SigniCcant Adverse Events PHASE 1 TRIAL IN SAFETY AND DOSING 15 Human safety to date » No clinically significant adverse events have been observed across 10 dosing cohorts Significance of clinical pharmacokinetics » High exposures at low oral dose, linearly dose proportional up to 175 mg. Exposures at 75 mg IkT - 148009 comparable to 500 mg imatinib 1 1 FDA summary data for approval 21 - 335 R² = 0.9977 R² = 0.9905 0 2 0 0 0 00 1 8 0 0 00 1 6 0 0 00 1 4 0 0 00 1 2 0 0 00 1 0 0 0 00 80000 60000 40000 20000 0 1 0 00 2 0 00 3 0 00 4 0 00 5 0 00 6 0 00 0 2 0 4 0 6 0 80 1 0 0 1 2 0 1 4 0 Oral Dose (mg) 1 6 0 1 8 0 Mean AUC 0 - ∞ (ng - h/mL Mean Cmax (ng) Clinical Pharmacokinetics of IkT - 148009 - SAD Cmax AUC Linear (Cmax) Linear (AUC)
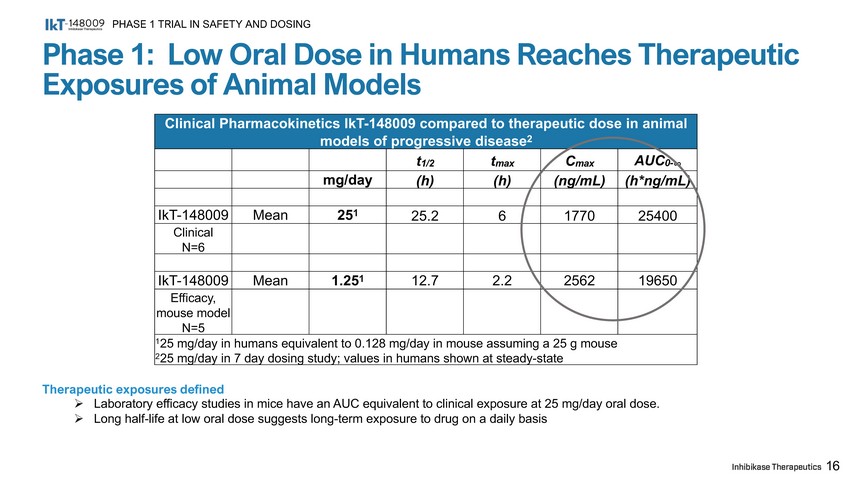
Phase 1: Low Oral Dose in Humans Reaches Therapeutic Exposures of Animal Models PHASE 1 TRIAL IN SAFETY AND DOSING 16 Clinical Pharmacokinetics IkT - 148009 compared to therapeutic dose in animal models of progressive disease 2 t 1/2 t max C max AUC 0 - ∞ mg/day (h) (h) (ng/mL) (h*ng/mL) I k T - 148009 Mean 25 1 25.2 6 1770 25400 Cli n ic a l N=6 I k T - 148009 Mean 1.25 1 12.7 2.2 2562 19650 Efficacy, mouse model N=5 1 25 mg/day in humans equivalent to 0.128 mg/day in mouse assuming a 25 g mouse 2 25 mg/day in 7 day dosing study; values in humans shown at steady - state Therapeutic exposures defined » Laboratory efficacy studies in mice have an AUC equivalent to clinical exposure at 25 mg/day oral dose. » Long half - life at low oral dose suggests long - term exposure to drug on a daily basis
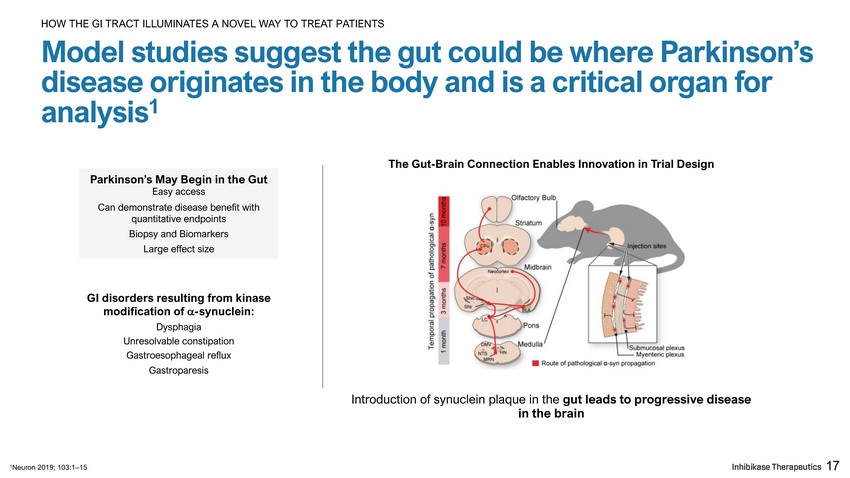
Parkinson’s May Begin in the Gut Easy access Can demonstrate disease benefit with quantitative endpoints Biopsy and Biomarkers Large effect size GI disorders resulting from kinase modification of - synuclein: Dysphagia Unresolvable constipation Gastroesophageal reflux Gastroparesis The Gut - Brain Connection Enables Innovation in Trial Design Introduction of synuclein plaque in the gut leads to progressive disease in the brain 17 Model studies suggest the gut could be where Parkinson’s disease originates in the body and is a critical organ for analysis 1 HOW THE GI TRACT ILLUMINATES A NOVEL WAY TO TREAT PATIENTS 1 Neuron 2019; 103:1 – 15
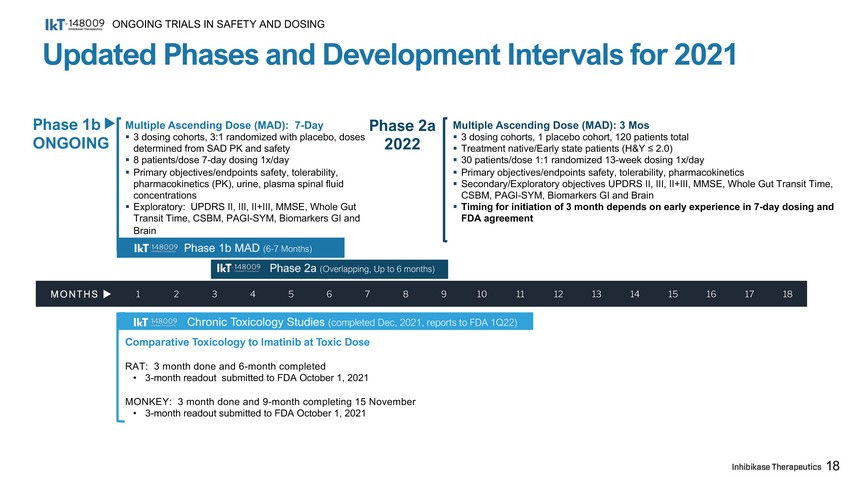
Updated Phases and Development Intervals for 2021 ONGOING TRIALS IN SAFETY AND DOSING Phase 1b MAD (6 - 7 Months) Multiple Ascending Dose (MAD): 7 - Day ▪ 3 dosing cohorts, 3:1 randomized with placebo, doses determined from SAD PK and safety ▪ 8 patients/dose 7 - day dosing 1x/day ▪ Primary objectives/endpoints safety, tolerability, pharmacokinetics (PK), urine, plasma spinal fluid concentrations ▪ Exploratory: UPDRS II, III, II+III, MMSE, Whole Gut Transit Time, CSBM, PAGI - SYM, Biomarkers GI and Brain Comparative Toxicology to Imatinib at Toxic Dose RAT: 3 month done and 6 - month completed • 3 - month readout submitted to FDA October 1, 2021 MONKEY: 3 month done and 9 - month completing 15 November • 3 - month readout submitted to FDA October 1, 2021 Multiple Ascending Dose (MAD): 3 Mos ▪ 3 dosing cohorts, 1 placebo cohort, 120 patients total ▪ Treatment native/Early state patients (H&Y ≤ 2.0) ▪ 30 patients/dose 1:1 randomized 13 - week dosing 1x/day ▪ Primary objectives/endpoints safety, tolerability, pharmacokinetics ▪ Secondary/Exploratory objectives UPDRS II, III, II+III, MMSE, Whole Gut Transit Time, CSBM, PAGI - SYM, Biomarkers GI and Brain ▪ Timing for initiation of 3 month depends on early experience in 7 - day dosing and FDA agreement 18 MONTHS u 1 2 Phase 2a (Overlapping, Up to 6 months) 3 4 5 6 7 8 9 10 11 Chronic Toxicology Studies (completed Dec, 2021, reports to FDA 1Q22) 12 13 14 15 16 17 18 Phase 1b ON G OI N G Phase 2a 2022
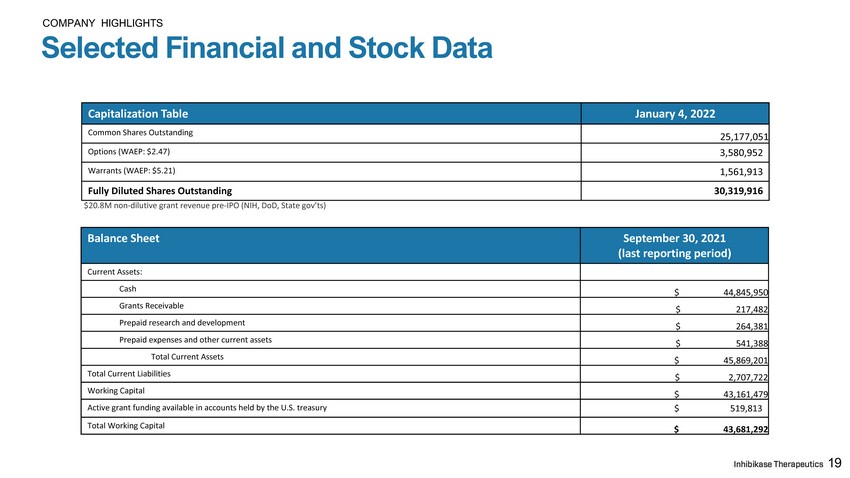
COMPANY HIGHLIGHTS Selected Financial and Stock Data 19 Balance Sheet September 30, 2021 (last reporting period) Current Assets: Cash $ 44 , 845 , 95 0 Grants Receivable $ 217 , 48 2 Prepaid research and development $ 264 , 38 1 Prepaid expenses and other current assets $ 541 , 38 8 Total Current Assets $ 45 , 869 , 20 1 Total Current Liabilities $ 2 , 707 , 72 2 Working Capital $ 43 , 161 , 47 9 Active grant funding available in accounts held by the U.S. treasury $ 519,813 Total Working Capital $ 43 , 681 , 29 2 $20.8M non - dilutive grant revenue pre - IPO (NIH, DoD, State gov’ts) Capitalization Table January 4, 2022 Common Shares Outstanding 25,177,051 Options (WAEP: $2.47) 3,580,952 Warrants (WAEP: $5.21) 1,561,913 Fully Diluted Shares Outstanding 30,319,916

Upcoming Milestones 20 COMPANY HIGHLIGHTS » 1Q22 • 148009 » Complete 250 mg SAD in healthy subjects » Complete two of three planned dosing cohorts in MAD 7 - day dosing in PD patients » Phase 2 study set - up in PD; first patient anticipated to commence in early 2Q22 » Complete first of two animal model validation studies of IkT - 148009 in MSA » Set - up EU/US sites for Phase 2 studies in MSA anticipated to commence in 3Q22 » Meet with FDA to review Phase 2 and Phase 3 development plans • 001Pro » File IND and commence bioequivalence clinical studies » Design and develop superiority studies for IkT - 001Pro relative to standard - of - care » Identify and begin developing commercial partnership

ABOUT US Management Team with Deep Experience in Drug Development and Commercialization Executive Milton Werner, PhD President & CEO Previously, Dr . Werner served as Director of Research at Celtaxsys . From September 1996 until June 2007 , Dr . Werner was a Head of the Laboratory of Molecular Biophysics at The Rockefeller University in New York City . Throughout his scientific career, Dr . Werner has been an innovator integrating chemistry, physics, and biology into a comprehensive approach to solving problems in medicine . Dr . Werner is the author or co - author of more than 70 research articles, reviews, and book chapters and has given lectures on his research work throughout the world . Joseph Frattaroli, CPA Chief Financial Officer Mr . Frattaroli is a certified public accountant with more than 15 years of experience in public company filings and compliance for Nasdaq and OTC Markets companies . Previously, he provided chief financial officer and consulting services for several emerging biopharmaceutical and medical device companies, with responsibilities that included capital formation, deal structuring, and assisting private companies in their transition to becoming publicly traded SEC registrants . C . Warren Olanow, MD, Interim Chief Medical Officer and Chief Executive Officer of CLINTREX . Dr . Olanow is the former Henry P . and Georgette Goldschmidt Professor and Chairman of the Department of Neurology at the Mount Sinai School of Medicine Prior to joining Mount Sinai, he served on the faculties of McGill University, Duke University, and the University of South Florida . He is the former President of the Movement Disorder Society, past President of the International Society of Motor Disturbances, and former Treasurer of the American Neurological Association . He has served on the executive committee of the Michael J . Fox Foundation Scientific Advisory Board, and he is the former Chairman of the Scientific Advisory Board of the Bachmann - Strauss Parkinson Foundation and of the Dystonia Foundation . Dr . Olanow is the former Co - Editor - in - Chief of the journal Movement Disorders . Dr . Olanow received his medical degree from the University of Toronto, performed his neurology training at the New York Neurological Institute at Columbia Presbyterian Medical Center at Columbia University, and undertook postgraduate studies in neuroanatomy at Columbia University and authored more than 600 articles in the field of neurodegeneration . 21

Board of Directors I n d u s t r y - L e a d i n g A d v i s o r s Mr . Dennis Berman • Co - founder, board member, and/or seed investor in many private biotechnology and technology companies, five of which have gone public . • Currently serves as the President of Molino Ventures, LLC a board advisory and venture capital firm and was co - founder and Executive Vice President of Corporate Development of Tocagen . • Seed investor, co - founder, and/or board member of Intervu, Kintera, Inc . , Gensia, Calabrian Dr. Roy Freeman, MD • Professor of Neurology at the Harvard Medical School and Director of the Center for Autonomic and Peripheral Nerve Disorders in the Department of Neurology at Beth Israel Deaconess Medical Center • Former chairman of the World Federation of Neurology research group on the autonomic nervous system, former President of the American Autonomic Society, and former chairman of the Autonomic Section of the American Academy of Neurology . • Editor - in - Chief of Autonomic Neuroscience : Basic and Clinical and on the editorial boards of The Clinical Journal of Pain, Pain : Clinical Updates, and Clinical Autonomic Research . • Serial founder of several companies in pain and neurodegenerative disease and is on the scientific advisory boards of many large and small pharmaceutical and biotechnology companies . Dr. Paul Grint, MD • 20 + years experience in biologics and small - molecule research and development, including the successful approval and commercialization of products in the infectious diseases, immunology, and oncology therapeutic areas . • Director of Amplyx Pharmaceuticals and Synedgen . • Served in senior management roles at Cerexa, Forest Laboratories, Kalypsys, Pfizer, IDEC Pharmaceuticals, and Schering - Plough Corporation . • Fellow of the Royal College of Pathologists and a medical degree from St . Bartholomew’s Hospital College, University of London . Ms. Elizabeth O’Farrell • 25 - year career with Eli Lilly and Company, lastly serving as Chief Procurement Officer and Leader, Global Head of Shared Services • Served in senior management at Lilly including Senior Vice President, Policy and Finance ; Senior Vice President, Finance ; Chief Financial Officer, Lilly USA ; Chief Financial Officer, Lilly Canada ; and General Auditor . Before joining Eli Lilly, Ms . • Director of PDL BioPharma, Geron Corporation and Lensar • BS in accounting with honors and an MBA in management information systems from Indiana University . Robert Hauser, MD Professor of Neurology, University of South Florida College of Medicine - Director USF Parkinson’s Disease and Movement Disorders Center Jeffrey Kordower, PhD Alla V and Solomon Jesmer Professor of Aging & Neurological Sciences Rush University Medical Center Dr. Ken Marek President and Senior Scientist, Institute of Neurodegenerative Disorders Dr. Ted Dawson, MD, PhD Neurodegeneration and Stem Cell Programs, Institute for Cell Engineering, Departments of Neurology, Physiology, Pharmacology, and Molecular Sciences - The Johns Hopkins University School of Medicine Dr. Valina Dawson, PhD Neurodegeneration and Stem Cell Programs, Institute for Cell Engineering, Departments of Neurology and Physiology The Johns Hopkins University School of Medicine Dr . Warren Olanow, MD, FRCPC Henry P . and Georgette Goldschmidt Professor and Chairman Emeritus, Mount Sinai School of Medicine Clintrex, Inc . Dr. Karl Kieburtz, MD, MPH Robert J. Joynt Professor in Neurology, Senior Associate Dean for Clinical Research, Director of the Clinical &Translational Science Institute, Founder Center for Human Experimental Therapeutics (CHET) - University of Rochester Medical Center Clintrex, Inc. Dr. Jay Pasricha, MBBS, MD Director, Johns Hopkins Center for Neurogastroenterology Professor of Medicine 22

Proof of the importance of c - Abl in Disease, Target Engagement and Functional Reversal in the Brain and Gut IKT Appendix
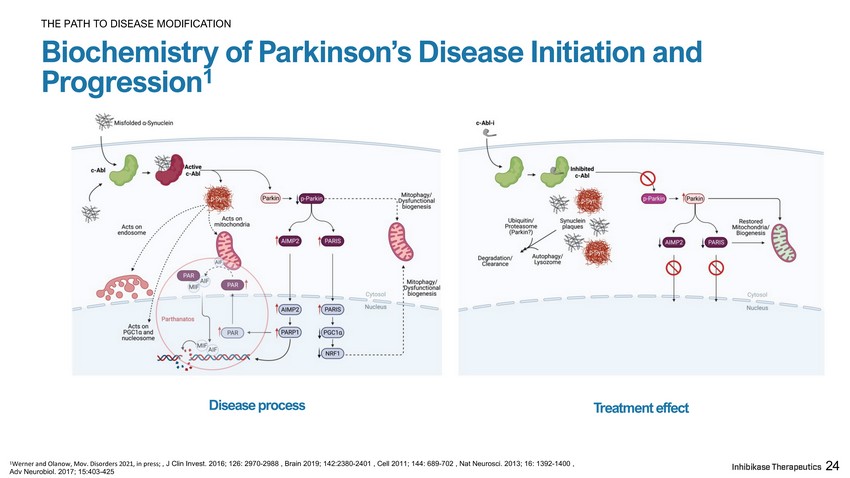
24 Biochemistry of Parkinson’s Disease Initiation and Progression 1 THE PATH TO DISEASE MODIFICATION 1 Werner and Olanow, Mov. Disorders 2021, in press; , J Clin Invest. 2016; 126: 2970 - 2988 , Brain 2019; 142:2380 - 2401 , Cell 2011; 144: 689 - 702 , Nat Neurosci. 2013; 16: 1392 - 1400 , Adv Neurobiol. 2017; 15:403 - 425 Disease process Treatment effect
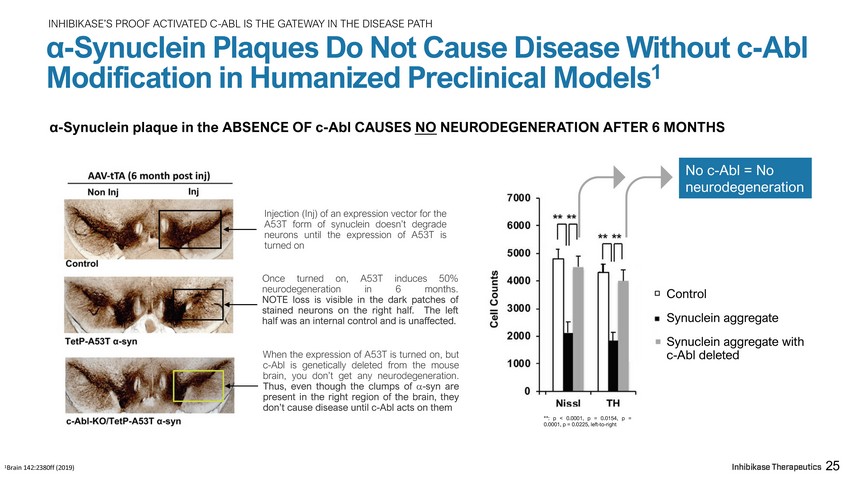
INHIBIKASE’S PROOF ACTIVATED C - ABL IS THE GATEWAY IN THE DISEASE PATH α - Synuclein Plaques Do Not Cause Disease Without c - Abl Modification in Humanized Preclinical Models 1 α - Synuclein plaque in the ABSENCE OF c - Abl CAUSES NO NEURODEGENERATION AFTER 6 MONTHS 1 Brain 142:2380ff (2019) Control Synuclein aggregate Synuclein aggregate with c - Abl deleted Injection (Inj) of an expression vector for the A 53 T form of synuclein doesn’t degrade neurons until the expression of A 53 T is turned on On c e t u r n e d on , A 53 T i n d u c es 50 % n e u r o d e g e n er at i on i n 6 mo n t h s . NOTE loss is visible in the dark patches of stained neurons on the right half. The left half was an internal control and is unaffected. When the expression of A 53 T is turned on, but c - Abl is genetically deleted from the mouse brain, you don’t get any neurodegeneration . Thus, even though the clumps of - syn are present in the right region of the brain, they don’t cause disease until c - Abl acts on them 25 No c - Abl = No n e u r ode g ener a t i on **: p < 0.0001, p = 0.0154, p = 0.0001, p = 0.0225, left - to - right
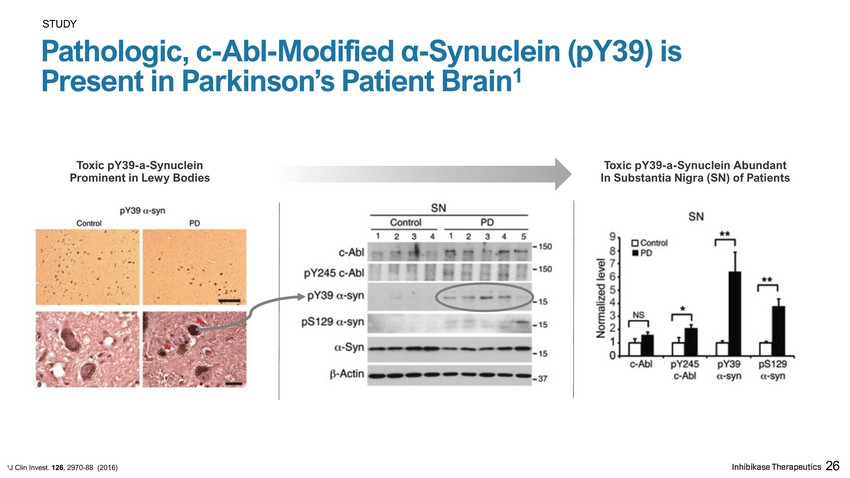
S T U D Y Pathologic, c - Abl - Modified α - Synuclein (pY39) is Present in Parkinson’s Patient Brain 1 Toxic pY39 - a - Synuclein Abundant In Substantia Nigra (SN) of Patients Toxic pY39 - a - Synuclein Prominent in Lewy Bodies 1 J Clin Invest. 126 , 2970 - 88 (2016) 26
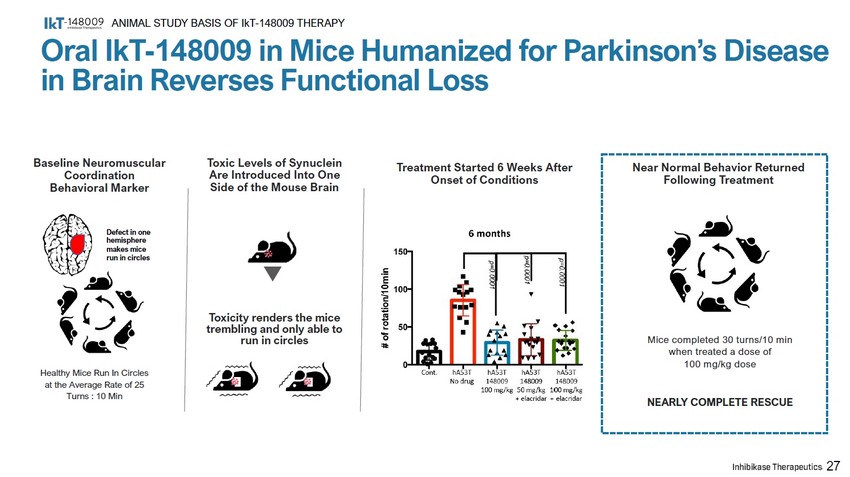
Baseline Neuromuscular Coordination Behavioral Marker Toxic Levels of Synuclein Are Introduced Into One Side of the Mouse Brain Healthy Mice Run In Circles at the Average Rate of 25 Turns : 10 Min Toxicity renders the mice trembling and only able to run in circles Treatment Started 6 Weeks After Onset of Conditions Near Normal Behavior Returned Following Treatment Mice completed 30 turns/10 min when treated a dose of 100 mg/kg dose NEARLY COMPLETE RESCUE 27 Defect in one hemisphere makes mice run in circles Oral IkT - 148009 in Mice Humanized for Parkinson’s Disease in Brain Reverses Functional Loss ANIMAL STUDY BASIS OF IkT - 148009 THERAPY
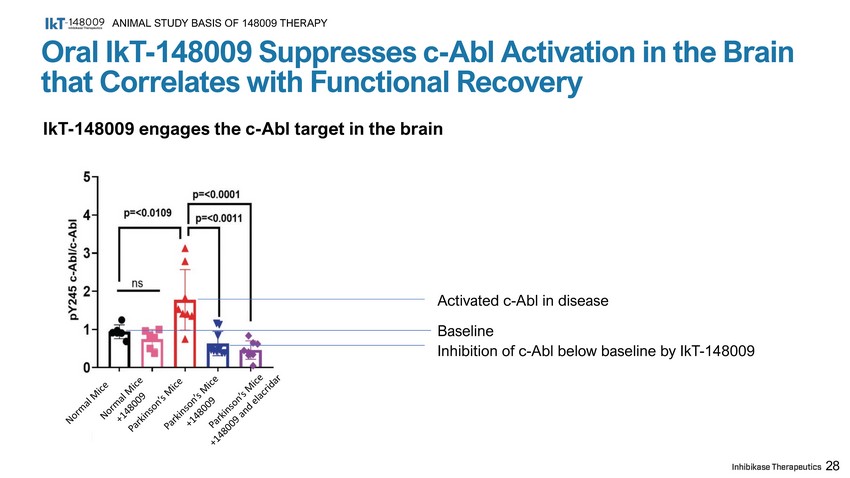
28 Oral IkT - 148009 Suppresses c - Abl Activation in the Brain that Correlates with Functional Recovery ANIMAL STUDY BASIS OF 148009 THERAPY IkT - 148009 engages the c - Abl target in the brain Activated c - Abl in disease Baseline Inhibition of c - Abl below baseline by IkT - 148009
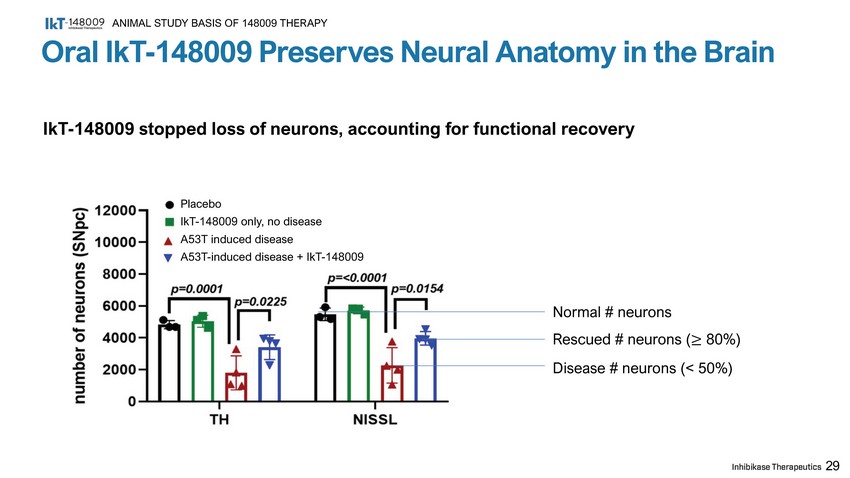
29 Placebo IkT - 148009 only, no disease A53T induced disease A53T - induced disease + IkT - 148009 Normal # neurons Rescued # neurons ( ≥ 80%) Disease # neurons (< 50%) Oral IkT - 148009 Preserves Neural Anatomy in the Brain ANIMAL STUDY BASIS OF 148009 THERAPY IkT - 148009 stopped loss of neurons, accounting for functional recovery
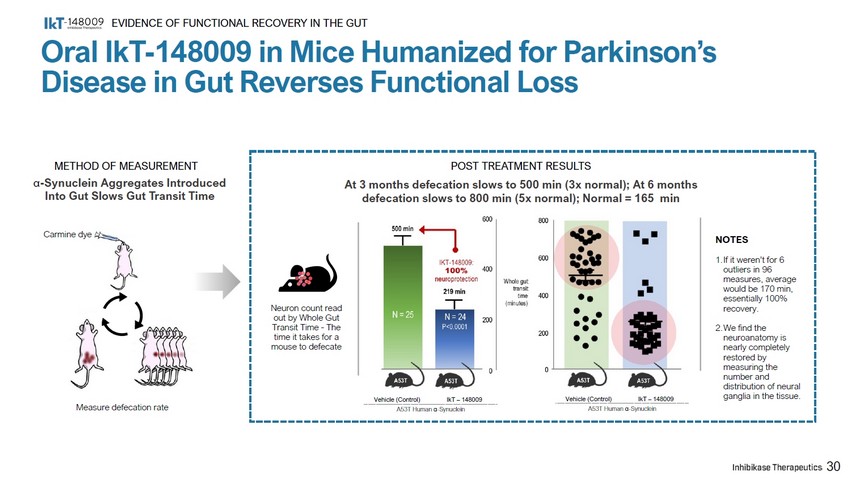
METHOD OF MEASUREMENT α - Synuclein Aggregates Introduced Into Gut Slows Gut Transit Time Measure defecation rate Neuron count read out by Whole Gut Transit Time - The time it takes for a mouse to defecate Carmine dye Vehicle (Control) IkT – 148009 Vehicle (Control) IkT – 148009 A53T Human α - Synuclein A53T Human α - Synuclein NOTES 1. If it weren’t for 6 outliers in 96 measures, average would be 170 min, essentially 100% recovery. 2. We find the neuroanatomy is nearly completely restored by measuring the number and distribution of neural ganglia in the tissue. POST TREATMENT RESULTS At 3 months defecation slows to 500 min (3x normal); At 6 months defecation slows to 800 min (5x normal); Normal = 165 min 30 Oral IkT - 148009 in Mice Humanized for Parkinson’s Disease in Gut Reverses Functional Loss EVIDENCE OF FUNCTIONAL RECOVERY IN THE GUT
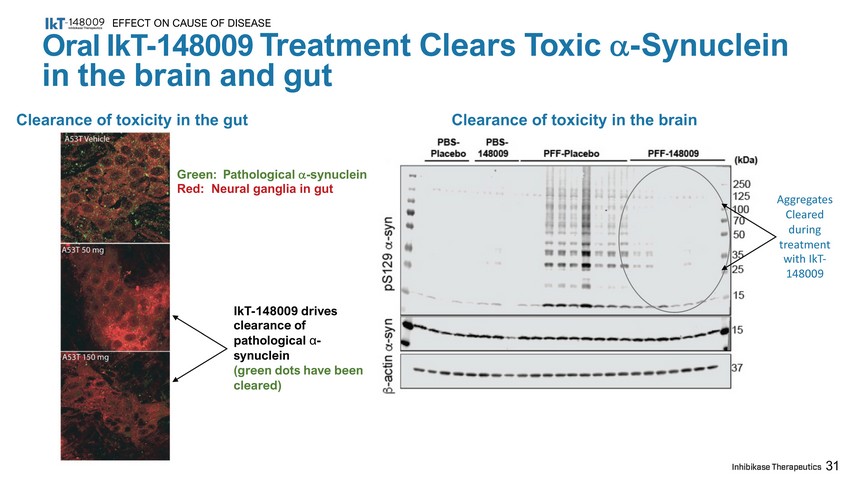
IkT - 148009 drives clearance of pathological α - synuclein (green dots have been cleared) 31 Oral IkT - 148009 Treatment Clears Toxic - Synuclein in the brain and gut EFFECT ON CAUSE OF DISEASE Clearance of toxicity in the gut Clearance of toxicity in the brain Green: Pathological - synuclein Red: Neural ganglia in gut A g g r e g at es Cleared during treatment with IkT - 148009
▪ IKT - 148009 drives functional recovery inside and outside of the brain ▪ IKT - 148009 drives clearance of the toxic form of - synuclein ▪ Low oral doses in humans achieve therapeutic exposure levels observed in animal efficacy studies Targeting c - Abl we believe is transformational to treatment of neurodegeneration 32 RESEARCH ADVANCES ARE TRANSFORMATIONAL Advances in pre - clinical models and clinical dosing